 A landmark study by Dale E. Bredesen, MD, was published last month in the journal Aging, showing the therapeutic value of a functional medicine approach to memory loss and cognitive decline related to neurodegenerative disease, such as Alzheimer’s disease (AD) on a small group of aging patients. This is the first study of this kind which examined the therapeutic benefit of making simple dietary changes, changes in sleep and physical activity levels, stress management counseling and individualized targeted nutritional supplementation to improve metabolic pathways involved in brain function based on physiologic imbalances identified in lab work on the participants. Sound familiar? This is the same approach we use in functional medicine on each and every one of our patients who come to us for help with chronic conditions, including neurodegenerative conditions.
A landmark study by Dale E. Bredesen, MD, was published last month in the journal Aging, showing the therapeutic value of a functional medicine approach to memory loss and cognitive decline related to neurodegenerative disease, such as Alzheimer’s disease (AD) on a small group of aging patients. This is the first study of this kind which examined the therapeutic benefit of making simple dietary changes, changes in sleep and physical activity levels, stress management counseling and individualized targeted nutritional supplementation to improve metabolic pathways involved in brain function based on physiologic imbalances identified in lab work on the participants. Sound familiar? This is the same approach we use in functional medicine on each and every one of our patients who come to us for help with chronic conditions, including neurodegenerative conditions.
 The design of this study allowed the researchers to perform assessments and identify potential pathological mechanisms involved in the progression of these participants’ cognitive conditions prior to intervention. The goal was to intervene in as many of these mechanisms as possible with targeted nutrients as well as dietary and lifestyle changes to support these metabolic interventions, and observe the effects on their memory and cognition. What was the outcome? 9 out of 10 patients (90%) showed subjective or objective improvements in cognition within 3 to 6 months of initiation of treatment! This is a significant improvement over the pharmaceutical drugs currently used to treat AD or mild cognitive impairment (MCI) which show temporary and marginal benefits at best.
The design of this study allowed the researchers to perform assessments and identify potential pathological mechanisms involved in the progression of these participants’ cognitive conditions prior to intervention. The goal was to intervene in as many of these mechanisms as possible with targeted nutrients as well as dietary and lifestyle changes to support these metabolic interventions, and observe the effects on their memory and cognition. What was the outcome? 9 out of 10 patients (90%) showed subjective or objective improvements in cognition within 3 to 6 months of initiation of treatment! This is a significant improvement over the pharmaceutical drugs currently used to treat AD or mild cognitive impairment (MCI) which show temporary and marginal benefits at best.
 “We’ve been studying the underlying mechanisms of neurodegeneration in the test tube and in transgenic mice for 25 years, and we came to the conclusion that there is an imbalance between the physiological processes that mediate plasticity in Alzheimer’s disease ― between synaptoblastic (synapse formation) and synaptoclastic (synapase breakdown) signaling — similar to what we see in osteoporosis, where osteoblastic signaling is chronically exceeded by osteoclastic signaling, resulting in bone loss,” principal investigator Dale Bredesen, MD, professor of neurology and director, Mary S. Easton Center for Alzheimer’s Disease Research, University of California, Los Angeles, told Medscape Medical News.
“We’ve been studying the underlying mechanisms of neurodegeneration in the test tube and in transgenic mice for 25 years, and we came to the conclusion that there is an imbalance between the physiological processes that mediate plasticity in Alzheimer’s disease ― between synaptoblastic (synapse formation) and synaptoclastic (synapase breakdown) signaling — similar to what we see in osteoporosis, where osteoblastic signaling is chronically exceeded by osteoclastic signaling, resulting in bone loss,” principal investigator Dale Bredesen, MD, professor of neurology and director, Mary S. Easton Center for Alzheimer’s Disease Research, University of California, Los Angeles, told Medscape Medical News.
Through this nutritional and lifestyle intervention, “it appears we can correct this network imbalance by tweaking it at multiple sites,” Dr. Bredesen added.
 Study Design and Results
Study Design and Results
A total of 10 patients with memory loss associated with either AD, mild cognitive impairment, or subjective cognitive impairment were recruited for the study. Each participant was instructed to follow a personalized intervention program tailored to address specific metabolic deficits identified on laboratory testing believed to be affecting the plasticity of the participant’s brain, causing memory loss. Nine of the 10 patients displayed subjective or objective improvement in cognition within 3 to 6 months of initiation of treatment. The single patient who failed to respond to the intervention had late-stage AD.
 Six participants had discontinued working or were struggling with their jobs at study outset because of memory problems. “All were able to return to work or continue working with improved performance, and improvements have been sustained,” said Dr. Bredesen. At the present time, one patient has been followed for 2.5 years from the initial presentation, and the patient continues to show “sustained and marked improvement.” Dr. Bredesen noted that the level of improved function required to work effectively is an important outcome of any successful therapeutic intervention.
Six participants had discontinued working or were struggling with their jobs at study outset because of memory problems. “All were able to return to work or continue working with improved performance, and improvements have been sustained,” said Dr. Bredesen. At the present time, one patient has been followed for 2.5 years from the initial presentation, and the patient continues to show “sustained and marked improvement.” Dr. Bredesen noted that the level of improved function required to work effectively is an important outcome of any successful therapeutic intervention.
Failure of Pharmaceutical Drugs for Cognitive Disorders
 From the original published article:
From the original published article:
“Drug development for neurodegenerative disease has been, arguably, the field of greatest failure of biomedical therapeutics development. Patients with acute illnesses such as infectious diseases, or with other chronic illnesses, such as cardiovascular disease, human immunodeficiency virus infection, and even cancer, have access to more effective therapeutic options than do patients with AD or other neurodegenerative diseases. In the case of AD, there is not a single therapeutic that exerts anything beyond a marginal, unsustained symptomatic effect, with little or no effect on disease progression. Furthermore, in the past decade alone, hundreds of clinical trials have been conducted for AD, at an aggregate cost of billions of dollars, without success. This has led some to question whether the approach taken to drug development for AD is an optimal one.
The past few decades of genetic and biochemical research have revealed an extensive network of molecular interactions involved in AD pathogenesis, suggesting that a network-based therapeutics approach, rather than a single target-based approach, may be feasible and potentially more effective for the treatment of cognitive decline due to Alzheimer’s disease.”
 A Systems Biology Approach to AD
A Systems Biology Approach to AD
Earlier mouse studies suggested that the pathophysiology involved in the development of AD (ie: beta-amyloid precursor protein (APP) signaling) involved many inputs (ie: inflammatory mediators, hormonal mediators, apolipoproteins and lipid metabolism factors, etc.) and suggested that the pathology of AD dictates a system or program-type of intervention rather than a single targeted agent. The researchers described the basic tenets of the therapeutic system as the following:
- The goal is not simply to normalize metabolic parameters but to optimize them.
- Based on the hypothesis that AD results from an imbalance in an extensive plasticity network, the therapy should address as many network components as possible.
- The underlying network has a threshold effect, such that once enough of the network components have been impacted, the disease process would be halted or reversed.
- The therapy is personalized, based on the laboratory values affecting the plasticity network.
- The program is time-dependent, so there is continued optimization over time.
- For each network component, the goal is to address it physiologically, as far upstream as possible.
 Optimizing Metabolic Parameters
Optimizing Metabolic Parameters
“Just as for other chronic illnesses such as atherosclerotic cardiovascular disease, the goal is not simply to normalize metabolic parameters, but rather to optimize them,” the investigators write. Lab markers associated with the pathophysiology of neurodegenerative disease and cognitive decline were evaluated with the goal of moving the markers into an ideal range (functional range), rather than the standard laboratory reference range with the goal of optimizing these markers.
For example, a typical lab range for homocysteine is less than 12 µmol/l, however this study’s goal was to keep this marker below 7 µmol/l (as we do in functional medicine), as research indicates an elevation above 7 µmol/l indicates compromised methylation status and increased risk of neurodegenerative disease. When homocysteine was found to be above 7 µmol/l, supplementation with methylcobalamin (vitamin B12) and folic acid (MTHF) to lower homocysteine was recommended. Another example is how 25-hydroxy vitamin D levels were interpreted. Standard lab ranges for 25-OH vitamin D are 30-100ng/ml, however, in this study, the goal was to move 25-OH vitamin D levels up to 50-100ng/ml as higher levels have been associated with improved brain function and decreased risk of dementia.
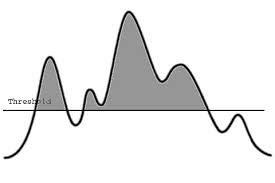 The Threshold Effect
The Threshold Effect
Critical to the success of this hypothesis is the idea that there is a “threshold” at which multiple interventions will start to reverse the pathology leading to memory loss. As Dr. Bredesen points out, it has been shown by Dean Ornish, MD, founder and president of Preventive Medicine Research Institute, San Francisco, California, among others that with a large enough lifestyle change, buildup of atherosclerotic plaque and subsequent coronary artery disease can be reversed. Similarly, in AD, if enough of the factors that contribute to the imbalance between synaptoblastic and synaptoclastic signaling in the brain can be reversed, deficits in the network that lead to memory loss can be redressed — “and you start to see improvement, which is exactly what we saw in these patients. If they follow enough of these interventions, they are able to improve,” Dr. Bredesen said.
“Based on the hypothesis that AD results from an imbalance in an extensive plasticity network, the therapy should address as many network components as possible, with the idea that a combination approach may create an effect that is more than the sum of the effects of many monotherapeutics,” the researchers add.
 Individualized Interventions
Individualized Interventions
The interventions used in the 10 patients involved in the UCLA pilot project were personalized to each individual just as we do in functional medicine, but they shared similar elements. Typically, patients were asked to eliminate all simple carbohydrates and processed foods from their diet and increase consumption of fruits, vegetables and wild fish (not farm-raised fish). They were also asked to avoid gluten (adopt a gluten-free diet) and follow a strict meal pattern with specifically timed interludes of fasting (12-hours). Exercise was a key component of all interventions, and participants were counseled on ways to reduce stress through practices such as yoga and meditation.
 Specific supplement recommendations were given to the participants depending on the results of the lab markers studied and symptomotology, such as vitamin D3, fish oils, methylcobalamin (vitamin B12), folic acid, curcumin, ashwagandha and probiotics. One of the main therapeutic principles for these cognitive patients was to reduce inflammation in the body and in the brain so many of these supplements were used for their studied anti-inflammatory effects, such as curcumin and fish oils. Optimizing antioxidants and mitochondrial function was emphasized so the use of mixed tocopherols (vitamin E), N-acetyl cysteine, alpha-lipoic acid, blueberries, vitamin C, coenzyme Q10, acetyl-L-carnitine, selenium, zinc and resveratrol were recommended. Hormonal imbalances in thyroid hormone, cortisol, and estrogen and progesterone were also addressed with the goal of optimizing hormone levels, just as we do in functional medicine.
Specific supplement recommendations were given to the participants depending on the results of the lab markers studied and symptomotology, such as vitamin D3, fish oils, methylcobalamin (vitamin B12), folic acid, curcumin, ashwagandha and probiotics. One of the main therapeutic principles for these cognitive patients was to reduce inflammation in the body and in the brain so many of these supplements were used for their studied anti-inflammatory effects, such as curcumin and fish oils. Optimizing antioxidants and mitochondrial function was emphasized so the use of mixed tocopherols (vitamin E), N-acetyl cysteine, alpha-lipoic acid, blueberries, vitamin C, coenzyme Q10, acetyl-L-carnitine, selenium, zinc and resveratrol were recommended. Hormonal imbalances in thyroid hormone, cortisol, and estrogen and progesterone were also addressed with the goal of optimizing hormone levels, just as we do in functional medicine.
“The program is not easy to follow,” Dr. Bredesen acknowledged. (None of the patients in this pilot project were able to fully follow the program). “But what this program says is that we are all contributing to our own AD by the diet we choose to eat; by the way we sleep; by the stress we have in our lives; by our microbiome; and of course by our genetics. The important thing here is, we can alter cognitive decline by affecting each of these parameters.”
 Growing Evidence
Growing Evidence
Commenting on the study for Medscape Medical News, Heather Snyder, PhD, director, medical and scientific relations, Alzheimer’s Association, Chicago, Illinois, said a number of studies have shown that lifestyle interventions can attenuate the progressive decline in cognitive function in older individuals.
Most recently, the FINGER study (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability), which was presented earlier this year at the Alzheimer’s Association International Conference 2014, showed that a multipronged lifestyle intervention had a significant beneficial effect on overall cognitive performance, including memory, executive function, and psychomotor speed, in a large cohort of older participants at high risk for cognitive decline.
 “The FINGER study certainly suggests that this is the kind of study we need to do in translating what Dr. Bredesen did to a much larger clinical trial,” Dr. Snyder said. Dr. Snyder also noted that it is clear the underlying pathology driving AD is already changing well before patients manifest overt memory loss and accompanying symptoms of the disease.
“The FINGER study certainly suggests that this is the kind of study we need to do in translating what Dr. Bredesen did to a much larger clinical trial,” Dr. Snyder said. Dr. Snyder also noted that it is clear the underlying pathology driving AD is already changing well before patients manifest overt memory loss and accompanying symptoms of the disease.
“This presents us with an opportunity to identify those individuals at the earliest stage of AD, when we can intervene with a medication or some type of nonpharmacological intervention,” she suggested.
 Important Features of Study
Important Features of Study
From my personal perspective, what makes this a landmark study is the design of the study. Each participant was put on an individualized dietary and nutritional protocol based on their lab markers and symptomotology. This follows the principles of functional medicine which appreciates the uniqueness of each individual’s genetics and unique physiology. This is rarely done in conventional medicine, especially in managed care, where each person is prescribed the “standard of care” which is usually a particular medication based on the person’s diagnosis, at the expense of all other considerations. Patients with the same diagnosis usually receive the same medication, with few exceptions. Genetic factors, family history, lab markers and other presenting symptoms are essentially ignored (unless they directly interfere or present a known problem with the medication).
 Another unique feature of this study was the multidimensional approach of the intervention. This intervention intentionally targeted multiple mechanisms simultaneously with the intention of impacting the “network” of pathophysiology that is involved in neurodegeneration and cognitive decline. The researchers called this a “threshold” that, if reached, could halt or even reverse the disease process. The concept used in this study was that if a sufficient number of biochemical pathways were targeted, then a threshold could be reached in which the pathophysiology underlying the disease process could be reversed. This is very different from the pharmaceutical approach in which usually only one or two mechanisms are targeted by the drug. These types of interventions are limited in their scope and therefore limited in their potential to have an impact on the underlying pathophysiology driving the disease process, which often involves multiple mechanisms.
Another unique feature of this study was the multidimensional approach of the intervention. This intervention intentionally targeted multiple mechanisms simultaneously with the intention of impacting the “network” of pathophysiology that is involved in neurodegeneration and cognitive decline. The researchers called this a “threshold” that, if reached, could halt or even reverse the disease process. The concept used in this study was that if a sufficient number of biochemical pathways were targeted, then a threshold could be reached in which the pathophysiology underlying the disease process could be reversed. This is very different from the pharmaceutical approach in which usually only one or two mechanisms are targeted by the drug. These types of interventions are limited in their scope and therefore limited in their potential to have an impact on the underlying pathophysiology driving the disease process, which often involves multiple mechanisms.
 The third distinguishing characteristic of this study was the use of dietary and lifestyle changes and nutritional supplements as the intervention. These are safe, low-cost interventions with minimal risk of side-effects and are easily obtained by practically anyone. Of course, the quality of the food and supplements used in the study can potentially impact the outcome. However, since no particular brand of food and supplements were recommended, these particular decisions were left to the individual and significant results were still obtained. It can be assumed that even better results are possible if higher quality food and supplements are used in the intervention.
The third distinguishing characteristic of this study was the use of dietary and lifestyle changes and nutritional supplements as the intervention. These are safe, low-cost interventions with minimal risk of side-effects and are easily obtained by practically anyone. Of course, the quality of the food and supplements used in the study can potentially impact the outcome. However, since no particular brand of food and supplements were recommended, these particular decisions were left to the individual and significant results were still obtained. It can be assumed that even better results are possible if higher quality food and supplements are used in the intervention.
 The fourth distinguishing characteristic, and most obvious, is the results obtained by the participants in the study. 90% of the participants showed subjective or objective improvements within a 3 to 6 month period. All 6 of the participants whose cognitive decline had a major impact on job performance were able to return to work or continue working with increased performance. This is a tremendous improvement over the medications currently approved for the use of these conditions and considered the current standard of care. The efficacy shown in reducing symptoms of cognitive decline and potentially reversing the neurodegenerative disease process by the use of dietary and lifestyle changes is truly astounding and holds much promise for the millions of sufferers of these conditions who until now have had few viable options.
The fourth distinguishing characteristic, and most obvious, is the results obtained by the participants in the study. 90% of the participants showed subjective or objective improvements within a 3 to 6 month period. All 6 of the participants whose cognitive decline had a major impact on job performance were able to return to work or continue working with increased performance. This is a tremendous improvement over the medications currently approved for the use of these conditions and considered the current standard of care. The efficacy shown in reducing symptoms of cognitive decline and potentially reversing the neurodegenerative disease process by the use of dietary and lifestyle changes is truly astounding and holds much promise for the millions of sufferers of these conditions who until now have had few viable options.
Original article published online in Aging, September 27, 2014. Full text of article
Excerpts of this article were taken from Medscape’s 10/3/2014 article, Novel Intervention May Reverse Alzheimer’s Memory Loss, by Pam Harrison.