 The Neuroendocrine System of the Gut
The Neuroendocrine System of the Gut
The neuroendocrine system (NES) of the gut consists of two parts: the gut endocrine cells, which are located in the gut mucosa, and the peptidergic, serotonergic and nitric oxide-containing nerves of the enteric nervous system (ENS) in the gut wall.[361] This system regulates several functions of the GI tract, such as motility, secretion, absorption, microcirculation in the gut, local immune defense and cell proliferation.[361] The ENS comprises a large variety of neurotransmitters and associated receptors. Almost every known neurotransmitter can be found in the ENS of the gut, and most of the receptors associated with these neurotransmitters are also found in the gut.[361]
 Gut Endocrine Cells
Gut Endocrine Cells
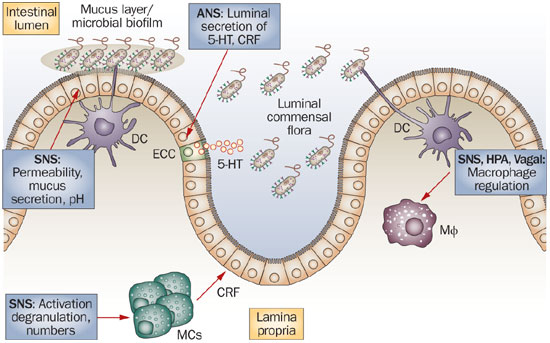
The gut contains a large number of endocrine cells that are dispersed among the epithelial cells of the gut mucosa throughout the entire GI tract except for the esophagus.[397-401] These cells constitute the largest endocrine organ in the body and comprise about 1% of all epithelial cells in the gastrointestinal tract, where they are separated from one another by epithelial cells.[397,398,402-404] These cells have specialized microvilli (tiny finger-like projections) that project into the lumen and function as sensors for the gut contents and respond to luminal stimuli by releasing hormones that, in general, target other parts of the digestive system.[405-414] There are at least 15 different populations of endocrine cells in the gastrointestinal tract.[397-399] Some of them have long slender cytoplasmic processes that project toward neighboring cells, increasing their paracrine effects (effects on surrounding cells).[415]
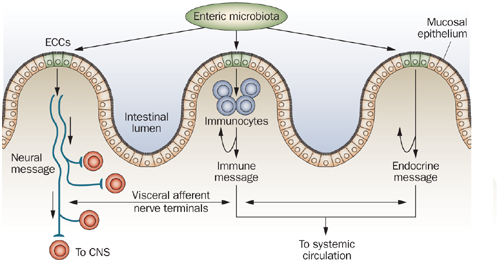 The gut endocrine cells secrete signaling substances into the lamina propria of the gut lining, where they exert their action locally on nearby structures (autocrine/paracrine function) and/or via an endocrine mode of action (by circulating in the blood to reach distant targets).[416] These cells interact in an integrated manner with each other and with the enteric nervous system (ENS) and the afferent and efferent nerve fibers of the central nervous system (CNS), in particular the autonomic nervous system.[399,417,418] These cells regulate several functions of the gastrointestinal tract, including sensation, motility, secretion, absorption, local immune defense, and even food intake (by affecting the appetite).[397-399,418]
The gut endocrine cells secrete signaling substances into the lamina propria of the gut lining, where they exert their action locally on nearby structures (autocrine/paracrine function) and/or via an endocrine mode of action (by circulating in the blood to reach distant targets).[416] These cells interact in an integrated manner with each other and with the enteric nervous system (ENS) and the afferent and efferent nerve fibers of the central nervous system (CNS), in particular the autonomic nervous system.[399,417,418] These cells regulate several functions of the gastrointestinal tract, including sensation, motility, secretion, absorption, local immune defense, and even food intake (by affecting the appetite).[397-399,418]
 Abnormalities in Gut Endocrine Cells in IBS Patients
Abnormalities in Gut Endocrine Cells in IBS Patients
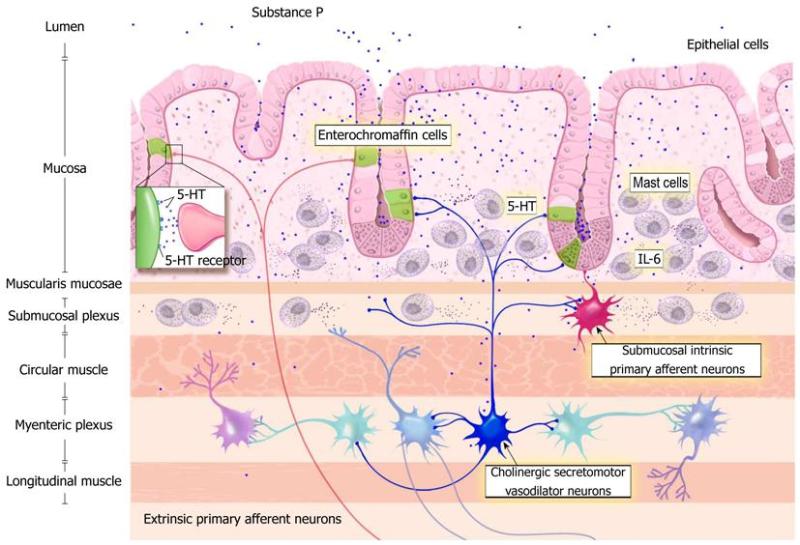
Abnormal endocrine/paracrine cells have been found in the stomach, proximal small intestine (duodenum), distal small intestine (ileum), colon and rectum of patients with IBS.[419-428] These gut endocrine cells produce various signaling peptides, such as grehlin, gastric inhibitory polypeptide, somatostatin, secretin, cholecystokinin (CCK), serotonin and polypeptide YY (PYY). Each of these peptides play important roles in secretion and gastric motility. In addition to the abnormalities observed in the endocrine cells, there are alterations in the levels of serotonin transporter (SERT), which appear to be increased in the ileum and decreased in the rectum of IBS patients.[428-430] Additionally, the mucosal 5-HT (serotonin) concentration has also been reported to be low in IBS patients which is significant since serotonin is the primary signaling hormone of the gut.[431]
 Is an Altered NES the Cause of IBS?
Is an Altered NES the Cause of IBS?
In a recent paper published in a world gastroenterology journal, Magdy, El-Salhe, et al. propose that these abnormalities observed in the gut endocrine cells may constitute an anatomical basis for the disturbances in digestion, GI motility and hypersensitivity problems seen in IBS.[360]
“In conclusion, there are sufficient grounds to suspect that the abnormalities in the gastrointestinal endocrine cells play a role in the development of visceral hypersensitivity, gastrointestinal dysmotility, and abnormal gastrointestinal secretion (in IBS patients).”[360]
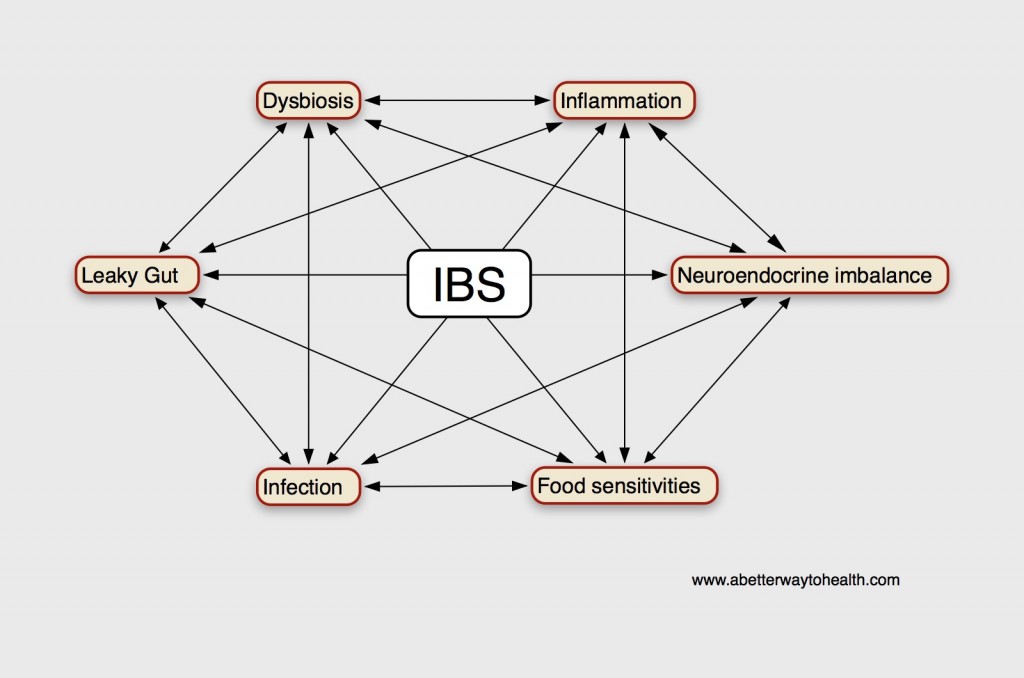 What Causes an Altered NES of the Gut?
What Causes an Altered NES of the Gut?
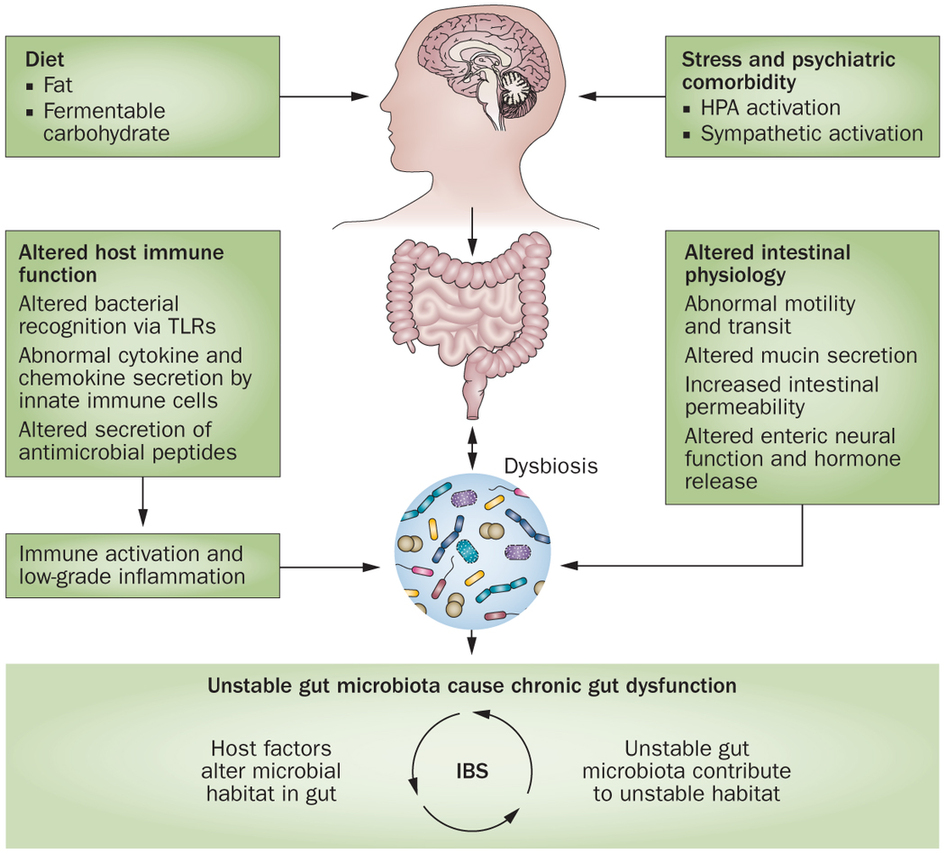
According to Magdy, El-Salhe, et al., the alteration in the NES could be the result of one or more of the following factors: genetic factors, dietary intake, intestinal flora or low-grade inflammation.[360] Genetic differences have been found between IBS patients and healthy subjects in genes controlling the serotonin signalling system and CCK. [422,425,426] Additionally, differences in the diet and the diversity and type of intestinal flora may affect the NES of the gut.[360] The release of different gut hormones depends on the composition and quantity of ingested food and intestinal bacteria present. The type and quantity of carbohydrate and fiber consumed, the amount of HCl and digestive enzymes present to aid in digestion and the subsequent fermentation of these unabsorbed foods by various intestinal bacteria can contribute to intestinal osmotic pressure (IAP). This change in intestinal pressure can stimulate hormonal release, such as the release of serotonin.[361]
 Likewise, inflammation and the release of secretory products from immune cells effects hormonal release and the proliferation of gut endocrine cells. Serotonin secretion, the predominant signaling hormone of the gut, can be enhanced or attenuated by the secretory products of certain immune cells such as CD4+ T-helper cells.[432] IBS has been associated with increased risk of inflammatory bowel disease (IBD) as well. IBS occurs in 32%-46% of patients with ulcerative colitis (UC) and in 42%-60% of Crohn’s disease patients who are in remission.[433-437] Fecal calprotectin has been found to be significantly elevated in UC and Crohn’s disease patients with IBS, compared to those without IBS-type symptoms, indicating the presence of GI inflammation.[437]
Likewise, inflammation and the release of secretory products from immune cells effects hormonal release and the proliferation of gut endocrine cells. Serotonin secretion, the predominant signaling hormone of the gut, can be enhanced or attenuated by the secretory products of certain immune cells such as CD4+ T-helper cells.[432] IBS has been associated with increased risk of inflammatory bowel disease (IBD) as well. IBS occurs in 32%-46% of patients with ulcerative colitis (UC) and in 42%-60% of Crohn’s disease patients who are in remission.[433-437] Fecal calprotectin has been found to be significantly elevated in UC and Crohn’s disease patients with IBS, compared to those without IBS-type symptoms, indicating the presence of GI inflammation.[437]
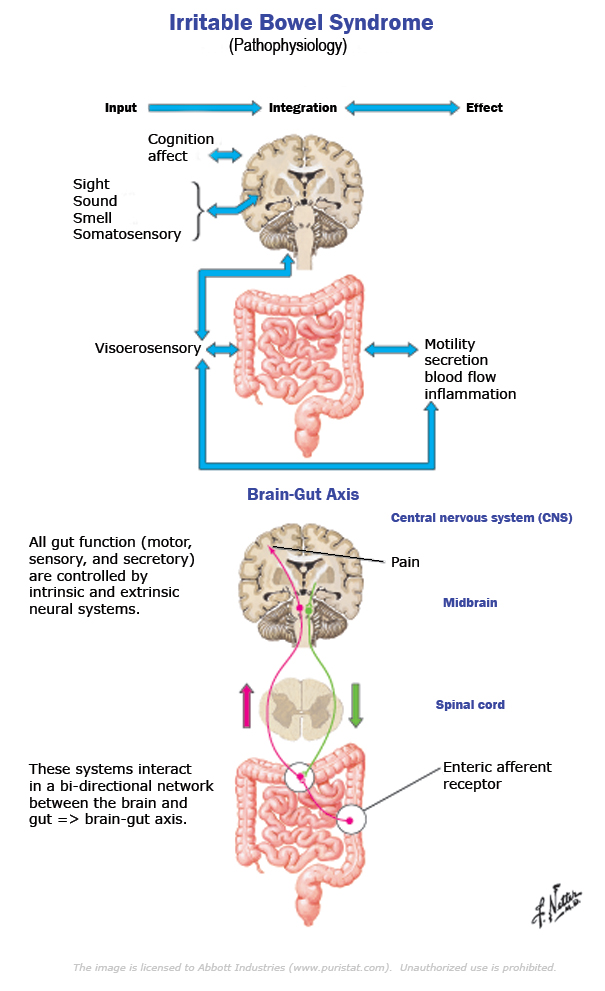 The Brain-Gut Axis
The Brain-Gut Axis
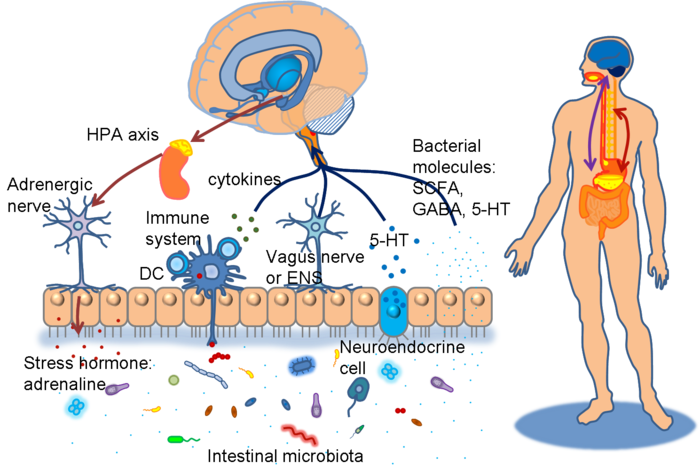
The NES of the gut is part of a larger system of communication between the brain and the gut that regulates all aspects of bowel activity and is gaining wider attention in the literature, known as the “brain-gut axis”. IBS is being viewed by many researchers in this field as a dysfunction of the brain-gut axis in the recent literature.[438-443]
“Alterations in the bi-directional signaling between the enteric nervous system and the central nervous system and consequently between the brain and the gut may play a significant role in the pathophysiology of IBS.”[438]
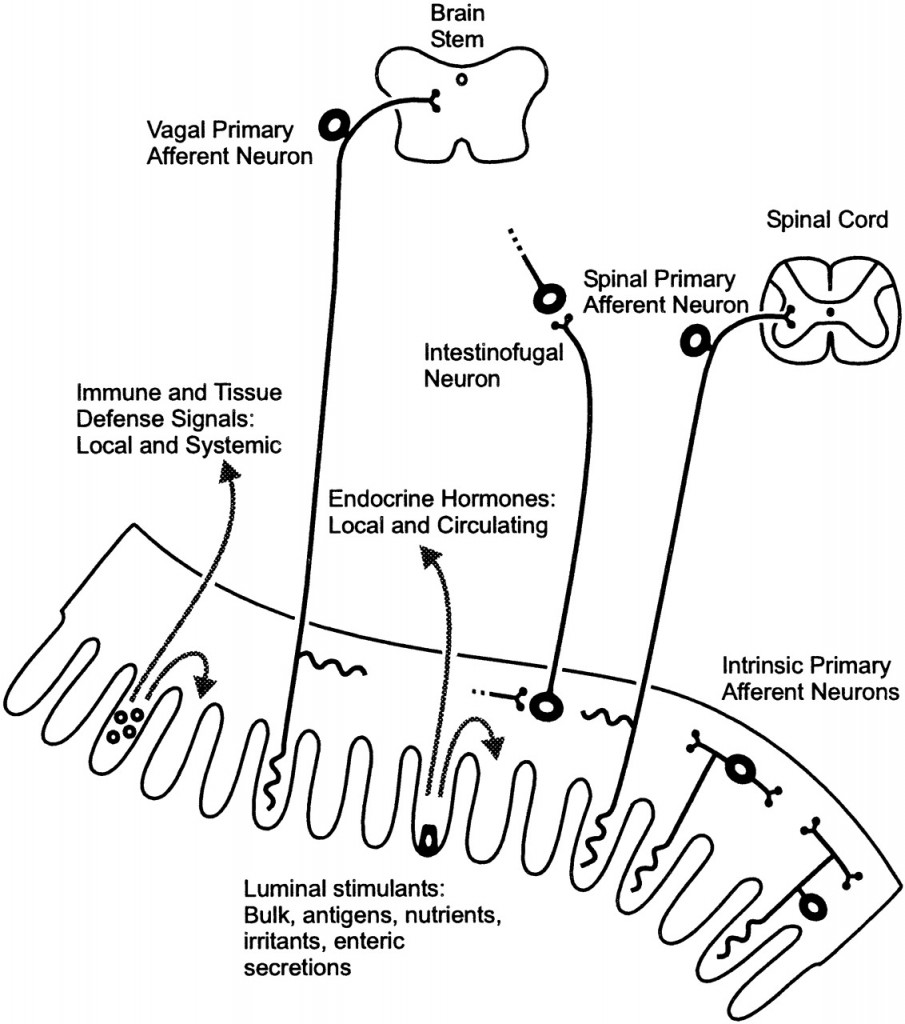
Communication between the brain and gut is complex and involves multiple systems including the brain cortex, hypothalamus, pituitary and adrenal glands. These structures are closely linked either by the peripheral nervous system or through neurohumoral stimuli and distinct responses have been shown in healthy subjects and IBS patients.[444,445] 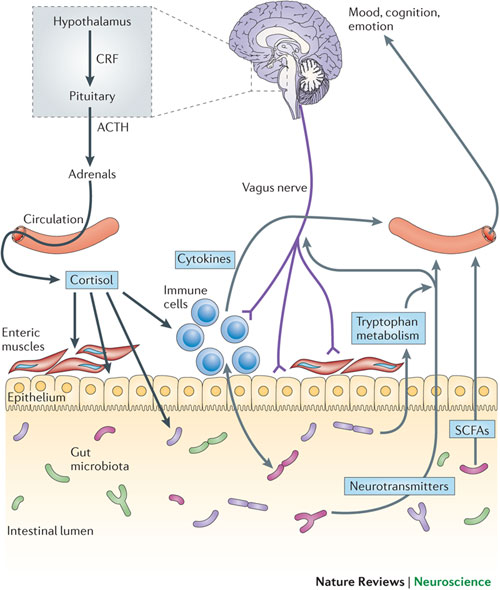 The gastrointestinal (GI) tract has a unique neuronal innervation that includes both an intrinsic neural network called the enteric nervous system (ENS) and an extrinsic neural network with connections to the brain via the spinal cord that provides sympathetic and parasympathetic innervation. The vagus nerve, an important part of the parasympathetic system, and its branches provide an important connection between the brain and the gut and convey both information to (ascending) and from (descending) the brain. These networks play a crucial role in the normal regulation and homeostasis of the GI tract.[438]
The gastrointestinal (GI) tract has a unique neuronal innervation that includes both an intrinsic neural network called the enteric nervous system (ENS) and an extrinsic neural network with connections to the brain via the spinal cord that provides sympathetic and parasympathetic innervation. The vagus nerve, an important part of the parasympathetic system, and its branches provide an important connection between the brain and the gut and convey both information to (ascending) and from (descending) the brain. These networks play a crucial role in the normal regulation and homeostasis of the GI tract.[438]
“There is evidence that interactions within the brain and gut axis (BGA) that involves both the afferent-ascending and the efferent-descending pathways as well as the somatosensory cortex, insula, amygdala, anterior cingulate cortex and hippocampus are deranged in IBS showing both activation and inactivation.”[438]
 Enteric Nervous System
Enteric Nervous System
The ENS is an autonomic network that can self-regulate itself and can function without input from the peripheral nervous system or brain.[446] It has been called “the brain of the gut” and “the second brain” based on its size, complexity and similarity to the brain.[447] This network is primarily housed in two nerve plexi and an intrinsic neural network. Its main function is to control movement of gut contents and peristalsis (coordinated muscle contractions of the bowel). It controls transit of intestinal contents through the stomach, small bowel and colon [448] and regulates secretory function and sensory-motor function.[446] These neurons are essential for the regulation of local reflexes that occur in response to various stimuli such as serotonin. Its activation results in motor and/or secretory responses.[449]
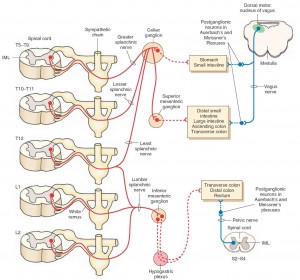 Extrinsic network
Extrinsic network
The extrinsic nerve supply to the GI tract modulates both secretion and motor function (and can override the neural signals of the ENS) and also establishes sensory communication between the ENS and the CNS. The extrinsic network acts in response to a broad variety of stimuli (thermal, chemical and painful) and via spinal and supra-spinal reflexes.[450] The extrinsic network is also comprised of two pathways: 1) Afferent (ascending): This involves vagal and spinal nerves that are mostly responsible for sensory transmission. 2) Efferent (descending): These pathways connect with both the peripheral and the brain, and provide information that induces both secretion of enzymes and peptides and induces peristalsis.[451] The CNS is a two-way traffic processor and regulator of bodily function.
“The interactions between the gut and the CNS are complex and a vital part of normal homeostasis and regulation of GI physiology. This is a delicately balanced and fine-tuned system that allows bidirectional relay of messages between the gut and the brain, both at rest and during provocation such as after a meal or luminal distension and controls movements, reflexes, and sensory perceptions in the gut.”[438]
 Stress, the HPA and Brain-Gut Axis
Stress, the HPA and Brain-Gut Axis
Stress has been linked to symptom generation in IBS and is associated with an altered response or a lack of adaptability in a subset of IBS patients.[452] Anxiety and depression are reported in 22%-30% of IBS patients and 32% have physical and/or sexual abuse.[453,454] Some IBS patients have a history of physical and/or sexual abuse causing traumatic stress which may lead to abnormal CNS pain processing.[455] There is some evidence showing that peripheral stimuli may activate stress hormones that may be involved in the pathophysiology of stress-sensitization. These types of IBS patients have increased psychological stress and increased severity of IBS symptoms. Treatment directed towards the psychiatric conditions can lead to reduction in symptom severity.[456]
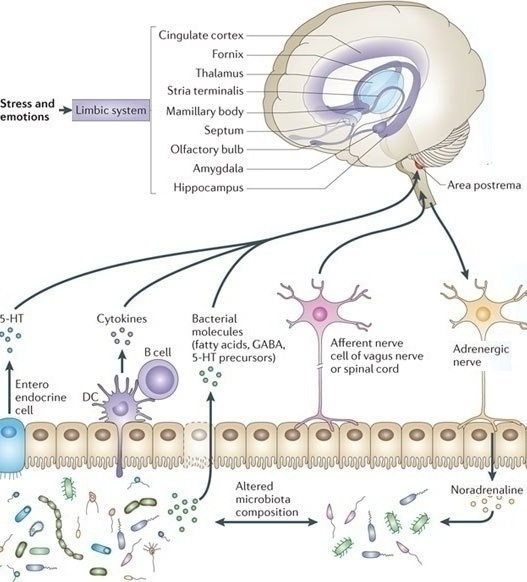 Neuroendocrine and autonomic output from the CNS to the periphery is modulated by several brain structures including the periventricular nucleus, amygdala and the pituitary gland.[457] Corticotropin (ACTH) plays a crucial role in regulating the response to stress. The limbic system through the amygdala is involved in the secretion of corticotropin releasing hormone (CRH or CRF), as well as the prefrontal cortex and anterior cingulate cortex.[458] Together they mediate the response to psychological stress through the hypothalamic-pituitary-adrenal (HPA) axis.[459]
Neuroendocrine and autonomic output from the CNS to the periphery is modulated by several brain structures including the periventricular nucleus, amygdala and the pituitary gland.[457] Corticotropin (ACTH) plays a crucial role in regulating the response to stress. The limbic system through the amygdala is involved in the secretion of corticotropin releasing hormone (CRH or CRF), as well as the prefrontal cortex and anterior cingulate cortex.[458] Together they mediate the response to psychological stress through the hypothalamic-pituitary-adrenal (HPA) axis.[459]
Studies in IBS patients have shown an impaired HPA axis. Anxiety and depression are also linked to alterations of the HPA axis. Emotion plays a key role in altering autonomic and endocrine function which in turn may derange the emotional circuitry. In healthy neural responses, peripheral stimuli travel through afferent pathways to the brain and they are filtered and evaluated in the subcortical regions before reaching the higher cortical regions of the brain, in order to prevent a circuit overload. When the filtering process is altered, stimuli can reach emotional areas, which in turn may activate deep brain structures leading to aberrant interpretation of symptoms. Consequently, non-noxious stimuli can be misinterpreted as painful or harmful. How this filtering process becomes altered in IBS patients is not yet known.[438]
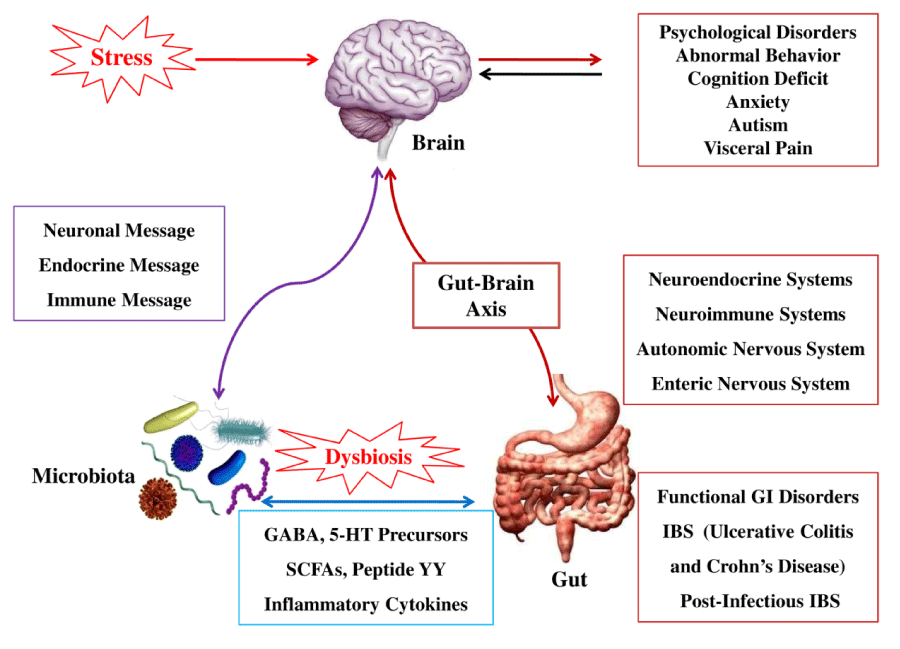
The Microbiota and the Brain-Gut Axis
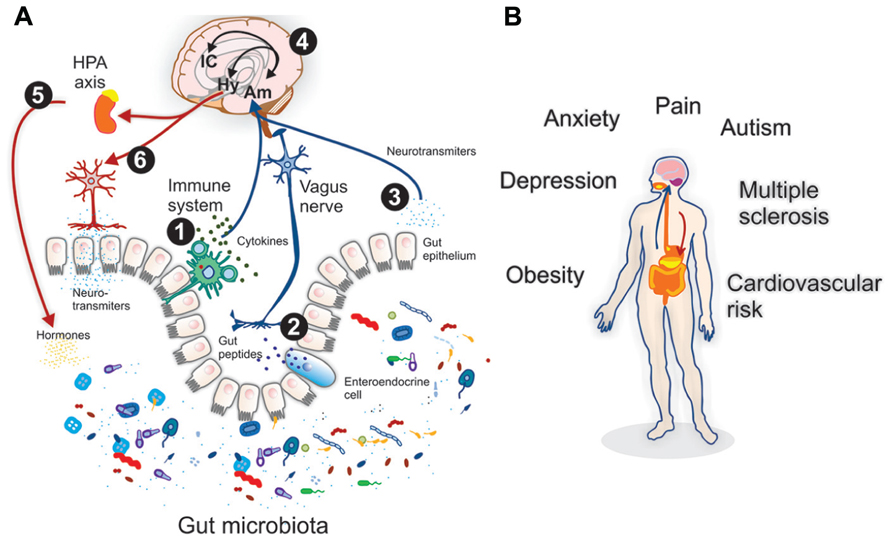
“The discovery of the size and complexity of the human microbiome has resulted in an ongoing reevaluation of many concepts of health and disease, including diseases affecting the CNS (brain). A growing body of preclinical literature has demonstrated bidirectional signaling between the brain and the gut microbiome, involving multiple neurocrine and endocrine signaling mechanisms.”[460]
The role that the intestinal microbiota play in the integrity of the brain-gut axis is recently gaining significant attention in the literature.[460-465] Several researchers are now referring to functional GI disorders (FGIDs), such as IBS, as a dysfunction of the microbiota-brain-gut axis.[461-464]
“A dysregulated gut-brain axis may be responsible for the main features of irritable bowel syndrome (IBS). However, the role of the gut microbiota is an underappreciated but critical node in this construct.”[461]
 Numerous studies have shown an association of dysbiosis (an imbalance of the gut flora) and IBS. One of the key questions that researchers are attempting to answer is whether dysbiosis is a cause of IBS.
Numerous studies have shown an association of dysbiosis (an imbalance of the gut flora) and IBS. One of the key questions that researchers are attempting to answer is whether dysbiosis is a cause of IBS.
“Numerous clinical studies have documented various alterations in the composition of the gut microbiota in IBS, indicating defects in stability and diversity of this virtual organ.”[461]
“Recently, attention has been focused on the microbiota as a possible factor in the pathogenesis of IBS.”[465]
 The gut microbiota have been shown to have an influence in several aspects to gut health, including immune responses, intestinal permeability, gut motility and abdominal pain. These are some of the ways that that the gut bacteria may contribute to the development of IBS.
The gut microbiota have been shown to have an influence in several aspects to gut health, including immune responses, intestinal permeability, gut motility and abdominal pain. These are some of the ways that that the gut bacteria may contribute to the development of IBS.
“It is clear that the microbiota is altered in IBS and that such alterations could well contribute to the pathogenesis of the disorder through, for example, increased permeability, an altered immune profile, effects on the central nervous system and modulation of gut neuromuscular function.”[465]
One of the important recent discoveries was that the symptoms associated with IBS can be improved by improving the flora of the gut.
“Manipulation of the gut microbiome influences the symptom profile in IBS and clear mechanisms have been elucidated to explain these interactions.”[464]
 Several specific bacterial probiotic strains have been shown to improve symptom severity and abdominal pain in IBS patients in numerous studies.[462,466-479] The symptoms that were assessed in the majority of these studies include abdominal pain, abdominal distension and bowel regularity. There are some species that in clinical trials appear to be more effective than others, such as Bifidobacterium species (B. infantis 3564 and B. bifidum MIMBb75) [470,474,476] and Lactobacillus species (Lactobacillus acidophilus-SDC 2012, 2013, L. paracasei B2106, L. plantarum 299V, and L. rhamnosus GG).[469,473,475] These probiotics appear to be effective in reducing abdominal pain and discomfort in adults and in children. Several studies have also suggested that combinations of different probiotic strains, such as VSL3# or mixtures of Bifidobacterium and Lactobacillus species, are able to decrease abdominal pain and discomfort in patients with IBS.[466,478,479]
Several specific bacterial probiotic strains have been shown to improve symptom severity and abdominal pain in IBS patients in numerous studies.[462,466-479] The symptoms that were assessed in the majority of these studies include abdominal pain, abdominal distension and bowel regularity. There are some species that in clinical trials appear to be more effective than others, such as Bifidobacterium species (B. infantis 3564 and B. bifidum MIMBb75) [470,474,476] and Lactobacillus species (Lactobacillus acidophilus-SDC 2012, 2013, L. paracasei B2106, L. plantarum 299V, and L. rhamnosus GG).[469,473,475] These probiotics appear to be effective in reducing abdominal pain and discomfort in adults and in children. Several studies have also suggested that combinations of different probiotic strains, such as VSL3# or mixtures of Bifidobacterium and Lactobacillus species, are able to decrease abdominal pain and discomfort in patients with IBS.[466,478,479]
“Accumulating data suggest that, in a significant percentage of patients, the microbiota plays an important role in the genesis and maintenance of FGIDs (ie: IBS). Probiotic supplementation appears to be of therapeutic value…”[462]
Gut Microbiota and Intestinal Permeability
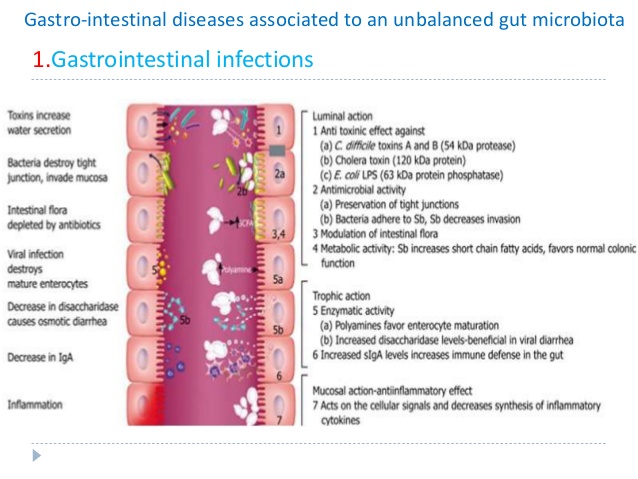 “Alterations in gut barrier function, occurring in response, or in parallel, to changes in the microbiota, have also been widely described and can be seen to play a pivotal role in generating and sustaining host immune responses both within and beyond the gut.”[469]
“Alterations in gut barrier function, occurring in response, or in parallel, to changes in the microbiota, have also been widely described and can be seen to play a pivotal role in generating and sustaining host immune responses both within and beyond the gut.”[469]
The gut microbiome has been shown to directly affect the epithelial barrier through its regulation of tight junction proteins.[465] Commensal flora also contribute to the production of mucus as the mucus layer is considerably reduced in the gut of germ-free mice, but recovers on exposure to bacterial products.[480,481] This may be important considering the compromised epithelial barrier and alterations in permeability observed in IBS.[482,483] The mechanisms underlying this increased permeability in IBS include alterations in tight junction protein function, changes in the microbiota, presence of active inflammation and/or presence of pro-inflammatory cytokines.[484] In particular, reduction  of the tight junction protein occludin-zonulin have been observed in IBS.[485,486]
of the tight junction protein occludin-zonulin have been observed in IBS.[485,486]
The authors of one article published in a major gastroenterology journal concluded the following regarding the role of the microbiota in IBS:
“Moreover, it has been reliably demonstrated that manipulation of the microbiota can influence the key symptoms, including abdominal pain and bowel habit, and other prominent features of IBS. There are clear mechanisms through which the microbiota can produce these effects, both humoral and neural. Taken together, these findings firmly establish the microbiota as a critical node in the gut-brain axis and one which is amenable to therapeutic interventions.”[464]
 Conclusion
Conclusion
An altered neuroendocrine system (NES) and dysregulation of the brain-gut axis appears to play a large role in the development of IBS. This is a new and exciting field of research with interesting theories and a number of potential directions of study. According to Magdy, El-Salhe, et al., the alteration in the gut NES could be the result of one or more of the following factors: genetic factors, dietary intake, intestinal flora or low-grade inflammation. As Magdy points out, the release of different gut hormones depends on the composition and quantity of ingested food. The type and quantity of carbohydrate and fiber consumed, the amount of HCl and digestive enzymes present to aid in digestion and the subsequent fermentation of these unabsorbed foods can contribute to intestinal osmotic pressure (IAP) which can stimulate hormonal release, such as the release of serotonin and contribute to the symptoms of IBS. This is the same mechanism involved in GERD and described in that previous section on GERD.
 As we will see in the next section on SIBO, there is a high percentage of prevalence of both IBS and bacterial overgrowth of the small intestines (SIBO) in the same individuals. This indicates a possible common mechanism between all three of these conditions. Namely, hypochlorhydria and/or excess carbohydrate intake leading to carbohydrate malabsorption and bacterial fermentation may be involved in all three of these common gut conditions.
As we will see in the next section on SIBO, there is a high percentage of prevalence of both IBS and bacterial overgrowth of the small intestines (SIBO) in the same individuals. This indicates a possible common mechanism between all three of these conditions. Namely, hypochlorhydria and/or excess carbohydrate intake leading to carbohydrate malabsorption and bacterial fermentation may be involved in all three of these common gut conditions.
Gut flora seem to play an important role in gut health and are directly affected by our diet, as the gut bacteria feed on food that is left undigested and unabsorbed.  Our diet has changed dramatically in recent years with the emergence and technological advances of the food industry, farming and agricultural changes. We now consume a large percentage of carbohydrates in the average diet and much of these are in the form of refined or processed carbohydrate and sugar, rather than whole grains, fruit and vegetables that may result in significant alterations in the balance of gut flora. In addition, our guts are being exposed to numerous environmental chemicals, hormones, antibiotics, genetically-modified foods and other toxins on a daily basis that may further affect the intestinal flora, intestinal permeability, intestinal inflammation and gut function.
Our diet has changed dramatically in recent years with the emergence and technological advances of the food industry, farming and agricultural changes. We now consume a large percentage of carbohydrates in the average diet and much of these are in the form of refined or processed carbohydrate and sugar, rather than whole grains, fruit and vegetables that may result in significant alterations in the balance of gut flora. In addition, our guts are being exposed to numerous environmental chemicals, hormones, antibiotics, genetically-modified foods and other toxins on a daily basis that may further affect the intestinal flora, intestinal permeability, intestinal inflammation and gut function.
In addition to diet, there are other important factors that play an important role as well in IBS, such as genetic factors, intestinal flora and inflammatory responses. There have also been other factors identified in IBS that likely contribute to this condition, including altered stress responses, abnormal levels of hormones involved in stress responses, an altered HPA axis and abnormal conduction and processing of neural stimuli. These factors likely contribute to the dysfunction of the brain-gut axis seen in IBS.
“Several mechanisms may be involved in the disruption and impairment of the brain and gut axis (BGA) that includes altered stress response, CRF release and processing, serotonin signaling and release, inflammatory insults and deranged conduction and processing of information”[438]
References
397. May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323: 70–75.[PMC free article] [PubMed]
398. Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–231. [PMC free article] [PubMed]
399. El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–2800. [PubMed]
400. Tanaka-Shintani M, Watanabe M. Immunohistochemical study of enterochromaffin-like cell in human gastric mucosa. Pathol Int. 2007;57:572–583. [PubMed]
401. Lönroth H, Håkanson R, Lundell L, Sundler F. Histamine containing endocrine cells in the human stomach. Gut. 1990;31:383–388. [PMC free article] [PubMed]
402. Buffa R, Capella C, Fontana P, Usellini L, Solcia E. Types of endocrine cells in the human colon and rectum. Cell Tissue Res. 1978;192:227–240. [PubMed]
403. Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. [PubMed]
404. Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed]
405. Sandström O, El-Salhy M. Ageing and endocrine cells of human duodenum. Mech Ageing Dev. 1999;108:39–48. [PubMed]
406. Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol. 2012;(209):309–335. [PubMed]
407. Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 2010;12:e1. [PubMed]
408. Raybould HE. Nutrient sensing in the gastrointestinal tract: possible role for nutrient transporters. J Physiol Biochem. 2008;64:349–356. [PubMed]
409. San Gabriel A, Nakamura E, Uneyama H, Torii K. Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J Med Invest. 2009;56 Suppl:209–217. [PubMed]
410. Rudholm T, Wallin B, Theodorsson E, Näslund E, Hellström PM. Release of regulatory gut peptides somatostatin, neurotensin and vasoactive intestinal peptide by acid and hyperosmolal solutions in the intestine in conscious rats. Regul Pept. 2009;152:8–12. [PubMed]
411. Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. [PMC free article] [PubMed]
412. Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007;292:G457–G461. [PubMed]
413. Buchan AM. Nutrient Tasting and Signaling Mechanisms in the Gut III. Endocrine cell recognition of luminal nutrients. Am J Physiol. 1999;277:G1103–G1107. [PubMed]
414. Montero-Hadjadje M, Elias S, Chevalier L, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem.2009;284:12420–12431. [PMC free article] [PubMed]
415. Sandström O. Age-related changes in the neuroendocrine system of the gut: A possible role in the pathogenesis of gastrointestinal diorders in the elderly. Umeå University Medical Dissertations. Umeå: Umeå; 1999. pp. 1–46. [PubMed]
416. Rindi G, Inzani F, Solcia E. Pathology of gastrointestinal disorders. Endocrinol Metab Clin North Am. 2010;39:713–727. [PubMed]
417. Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. [PMC free article] [PubMed]
418. Seim I, El-Salhy M, Hausken T, Gundersen D, Chopin L. Ghrelin and the brain-gut axis as a pharmacological target for appetite control. Curr Pharm Des. 2012;18:768–775. [PubMed]
419. El-Salhy M, Wendelbo IH, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241–1244. [PubMed]
420. El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508–3513. [PubMed]
421. El-Salhy M, Mazzawi T, Gundersen D, Hausken T. Chromogranin A cell density in the rectum of patients with irritable bowel syndrome. Mol Med Rep. 2012;6:1223–1225. [PMC free article] [PubMed]
422. El-Salhy M, Lomholt-Beck B, Hausken T. Chromogranin A as a possible tool in the diagnosis of irritable bowel syndrome. Scand J Gastroenterol. 2010;45:1435–1439. [PubMed]
423. El-Salhy M, Lillebø E, Reinemo A, Salmelid L. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23:703–707. [PubMed]
424. El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–878. [PMC free article] [PubMed]
425. Sjölund K, Ekman R, Wierup N. Covariation of plasma ghrelin and motilin in irritable bowel syndrome. Peptides. 2010;31:1109–1112. [PubMed]
426. Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. [PMC free article] [PubMed]
427. Park JH, Rhee PL, Kim G, et al. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–546. [PubMed]
428. Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. [PubMed]
429. El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–455. [PubMed]
430. Wendelbo I, Mazzawi T, El-Salhy M. Increased serotonin transporter immunoreactivity intensity in the ileum of patients with irritable bowel disease. Mol Med Rep. 2014;9: 180–184; Epub 2013 Nov 8. [PubMed]
431. El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci.2012;57:873–878. [PMC free article] [PubMed]
432. Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. [PMC free article] [PubMed]
433. Isgar B, Harman M, Kaye MD, Whorwell PJ. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut. 1983;24:190–192. [PMC free article] [PubMed]
434. Ansari R, Attari F, Razjouyan H, Etemadi A, Amjadi H, Merat S, Malekzadeh R. Ulcerative colitis and irritable bowel syndrome: relationships with quality of life. Eur J Gastroenterol Hepatol. 2008;20:46–50.[PubMed]
435. Simrén M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS-like symptoms and associated psychological factors. Am J Gastroenterol. 2002;97:389–396. [PubMed]
436. Minderhoud IM, Oldenburg B, Wismeijer JA, van Berge Henegouwen GP, Smout AJ. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci. 2004;49:469–474. [PubMed]
437. Keohane J, O’Mahony C, O’Mahony L, O’Mahony S, Quigley EM, Shanahan F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: a real association or reflection of occult inflammation? Am J Gastroenterol. 2010;105:1788, 1789–1794; quiz 1795. [PubMed]
438. Coss-Adame E, Rao SS. Brain and gut interactions in irritable bowel syndrome: new paradigms and new understandings. Curr Gastroenterol Rep. 2014 Apr;16(4):379. doi: 10.1007/s11894-014-0379-z. Review.
439. Emeran A. Mayer. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. Author manuscript; available in PMC 2013 December 2. Published in final edited form as: Nat Rev Neurosci. 2011 July 13; 12(8): 10.1038/nrn3071. Published online 2011 July 13. doi: 10.1038/nrn3071
440. Daulatzai MA. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem Res. 2014 Apr;39(4):624-44. doi: 10.1007/s11064-014-1266-6. Epub 2014 Mar 4. Review.
441. Yu Y, Wu S, Li J, Wang R, Xie X, Yu X, Pan J, Xu Y, Zheng L. The effect of curcumin on the brain-gut axis in rat model of irritable bowel syndrome: involvement of 5-HT-dependent signaling. Metab Brain Dis. 2015 Feb;30(1):47-55. doi: 10.1007/s11011-014-9554-z. Epub 2014 May 8.
442. Peter Holzer, Florian Reichmann, Aitak Farzi. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut–brain axis. Neuropeptides. 2012 December; 46(6): 261–274. doi: 10.1016/j.npep.2012.08.005
443. Hyland NP, O’Mahony SM, O’Malley D, O’Mahony CM, Dinan TG, Cryan JF.
Early-life stress selectively affects gastrointestinal but not behavioral responses in a genetic model of brain-gut axis dysfunction. Neurogastroenterol Motil. 2015 Jan;27(1):105-13. doi: 10.1111/nmo.12486. Epub 2014 Dec 2.
444. Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869.[PMC free article] [PubMed]
445. Straub RH, Herfarth H, Falk W, et al. Uncoupling of the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis in inflammatory bowel disease? J Neuroimmunol. 2002;126:116–125.[PubMed]
446. Hansen MB. The enteric nervous system I: The organization and classification. Pharmacol & Toxicol. 2003;92:105–113. [PubMed]
447. Gershon MD. The Second Brain. Harper Collins; New York: 1998.
448. Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96.[PubMed]
449. Langley JN. The Autonomic Nervous System, Part 1. Cambridge: W. Heffer; 1921.
450. Hansen MD. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52(1):1–30. [PubMed]
451. Hansen MB. The enteric nervous system II: Gastrointestinal functions. Pharmacol & Toxicol. 2003;92:249–257. [PubMed]
452. Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology.2006;130:1377–1390. [PubMed]
453. Thijssen AY, Jonkers DM, Leue C, et al. Dysfunctional cognitions, anxiety and depression in irritable bowel syndrome. J Clin Gastroenterol. 2010;44:e236–241. [PubMed]
454. Delvaux M, Denis P, Allemand H. Sexual abuse is more frequently reported by IBS patients than by patients with organic digestive diseases or controls. Results of a multicentre inquiry. French Club of Digestive Motility. Eur J Gastroenterol Hepatol. 1997;9:345–352. [PubMed]
455. Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–833. [PubMed]
456. Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–261. [PMC free article] [PubMed]
457. Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992;117:854–866. [PubMed]
458. Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38:225–234. [PubMed]
459. Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology.1983;36:165–186. [PubMed]
460. Emeran A. Mayer, Rob Knight, Sarkis K. Mazmanian, John F. Cryan, Kirsten Tillisch. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J Neurosci. 2014 November 12; 34(46): 15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014
461. Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014 Oct 21;20(39):14105-25. doi: 10.3748/wjg.v20.i39.14105.
462. De Palma G, Collins SM, Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes. 2014 May-Jun;5(3):419-29. doi: 10.4161/gmic.29417. Epub 2014 Jun 12.
463. Peter Holzer, Aitak Farzi. Neuropeptides and the Microbiota-Gut-Brain Axis.
Adv Exp Med Biol. Author manuscript; available in PMC 2015 March 15. Published in final edited form as: Adv Exp Med Biol. 2014; 817: 195–219. doi: 10.1007/978-1-4939-0897-4_9
464. Emeran A. Mayer, Tor Savidge, Robert J. Shulman. Brain Gut Microbiome Interactions and Functional Bowel Disorders. Gastroenterology. Author manuscript; available in PMC 2014 July 29. Published in final edited form as: Gastroenterology. 2014 May; 146(6): 1500–1512. Published online 2014 February 28. doi: 10.1053/j.gastro.2014.02.037
465. Hyland NP, Quigley EM, Brint E. Microbiota-host interactions in irritable bowel syndrome: epithelial barrier, immune regulation and brain-gut interactions. World J Gastroenterol. 2014 Jul 21;20(27):8859-66. doi: 10.3748/wjg.v20.i27.8859. Review.
466. Cui S, Hu Y. Multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Int J Clin Exp Med. 2012;5:238–44. [PMC free article][PubMed]
467. Diop L, Guillou S, Durand H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial. Nutr Res. 2008;28:1–5. doi: 10.1016/j.nutres.2007.10.001. [PubMed] [Cross Ref]
468. Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol. 2009;47:209–14. doi: 10.1055/s-2008-1027702. [PubMed] [Cross Ref]
469. Francavilla R, Miniello V, Magistà AM, De Canio A, Bucci N, Gagliardi F, Lionetti E, Castellaneta S, Polimeno L, Peccarisi L, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics. 2010;126:e1445–52. doi: 10.1542/peds.2010-0467. [PubMed] [Cross Ref]
470. Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–32. doi: 10.1111/j.1365-2036.2011.04633.x. [PubMed] [Cross Ref]
471. Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33:1302–10. doi: 10.1111/j.1365-2036.2011.04665.x. [PubMed] [Cross Ref]
472. Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med. 2009;121:119–24. doi:10.3810/pgm.2009.03.1984. [PubMed] [Cross Ref]
473. Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–7. doi: 10.1097/00042737-200110000-00004. [PubMed] [Cross Ref]
474. O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050.[PubMed] [Cross Ref]
475. Sinn DH, Song JH, Kim HJ, et al. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53:2714–8. doi: 10.1007/s10620-007-0196-4. [PubMed] [Cross Ref]
476. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x.[PubMed] [Cross Ref]
477. Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, Corfe BM. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [PubMed] [Cross Ref]
478. Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52–9. doi: 10.1111/jgh.12322. [PubMed] [Cross Ref]
479. Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46:220–7. doi: 10.1097/MCG.0b013e31823712b1. [PubMed] [Cross Ref]
480. Petersson J, Schreiber O, Hansson GC, et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G327–G333. [PMC free article] [PubMed]
481. Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298:G807–G819. [PubMed]
482. Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. 2013;69:42–51. [PubMed]
483. Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. [PubMed]
484. Frese SA, Benson AK, Tannock GW, et al. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 2011;7:e1001314. [PMC free article] [PubMed]
485. Bertiaux-Vandaële N, Youmba SB, Belmonte L, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106:2165–2173. [PubMed]
486. Martínez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. [PubMed]