In the previous article, we discussed how this novel coronavirus is generally thought to spread from person-to-person through respiratory droplets. These droplets may land directly on another person via sneezing, coughing or even breathing. They can also spread via contact with a contaminated object (doorknob) or surface (cardboard box or countertop) followed by touching one’s face, allowing viral entry through the nose, mouth, or even the eyes. If breathed in or ingested, the virus can enter the body via the respiratory tract (nose, throat, lungs) or the digestive tract (mouth, throat). Both the respiratory and digestive tracts are lined with epithelial cells, making up the epithelial lining of these organ systems.
The Mucosal Immune System
On entry into one of these epithelial-lined tracts, the virus will try to bind to the epithelial cells of the throat, mouth, and nose which line these cavities. However, the mucosal immune system protects the human body with a defensive barrier composed of a mucus layer and secretory IgA, which protect these epithelial cells. The mucus layer is the immune system’s first line of defense. Its job is to intercept the virus and prevent it from binding to epithelial cells. People with a healthy functioning mucosal immune system will produce a significant amount of mucus that will wash off the virus so that it does not bind to the epithelial cells, as shown below.
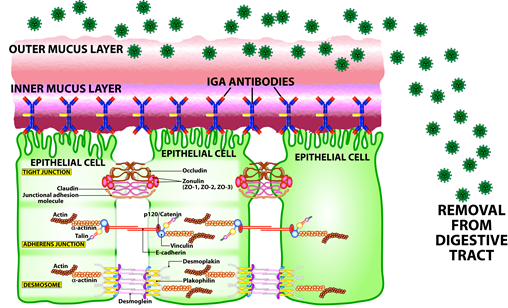
Secretory IgA
In the inner layer of mucus, immune cells (B cells and plasma cells) produce antibodies known as secretory IgA which bind to the internal surface of epithelial cells. Secretory IgA is the principal weapon protecting us from pathogens and toxins that might otherwise penetrate mucosal surfaces. These antibodies are key components of the mucosal mucus and other body secretions such as saliva and tears. It is important to appreciate how prominent secretory IgA is in immune defense. (Mucosal Illustrations provided by Aristo Vojdani, Ph.D. and Cyrex Labs)
Think of secretory IgA as the body’s homeland security. The job of secretory IgA is to prevent hostile viruses from breaking through the body’s defenses. It does this by blocking the virus and stopping it from binding to the epithelial receptors. Individuals with high levels of secretory IgA will be more protected than those with low secretory IgA. This is why many people may come in contact with the virus and still not get infected, if they have high levels of mucus and secretory IgA. However, low levels of mucus and secretory IgA make it easier for the coronavirus to bind to the epithelial cell and then enter the cell. This is likely part of the reason a small percentage of the population develop full-blown COVID-19 while others do not. Unfortunately, as part of aging, due to malnutrition and a decrease in mucus secretions, elderly people do not produce as much secretory IgA as the youth, and thus are more prone to viral infection in the respiratory tract.
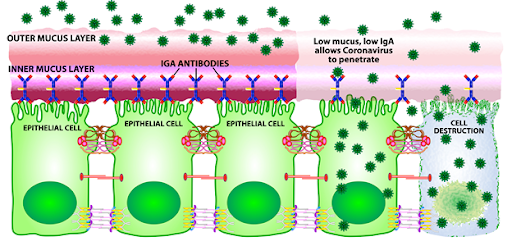
Decreased sIgA is very common in modern society and in my patient population as well. An important cause of decreased sIgA is chronic stress from elevated cortisol so it is not surprising that this is so common. Elevated cortisol from chronic stress suppresses both mucus production and production of sIgA. Stress responses can be assessed via salivary cortisol testing that measures levels of salivary cortisol. Some of these tests also include measurements of sIgA.
Viral Replication
Once within the epithelial cell, the virus will use the cell’s genetic machinery to reproduce and multiply inside the cell leading to cell destruction and the newly-formed viruses spreading to neighboring cells, binding to cell receptors, entering the cells and starting the process of viral replication and cell destruction all over again. However, even if the lack of a protective layer should allow the virus to penetrate, infect and replicate, not every person will show symptomatology. This is due to the next level of protection provided by the innate immune system.
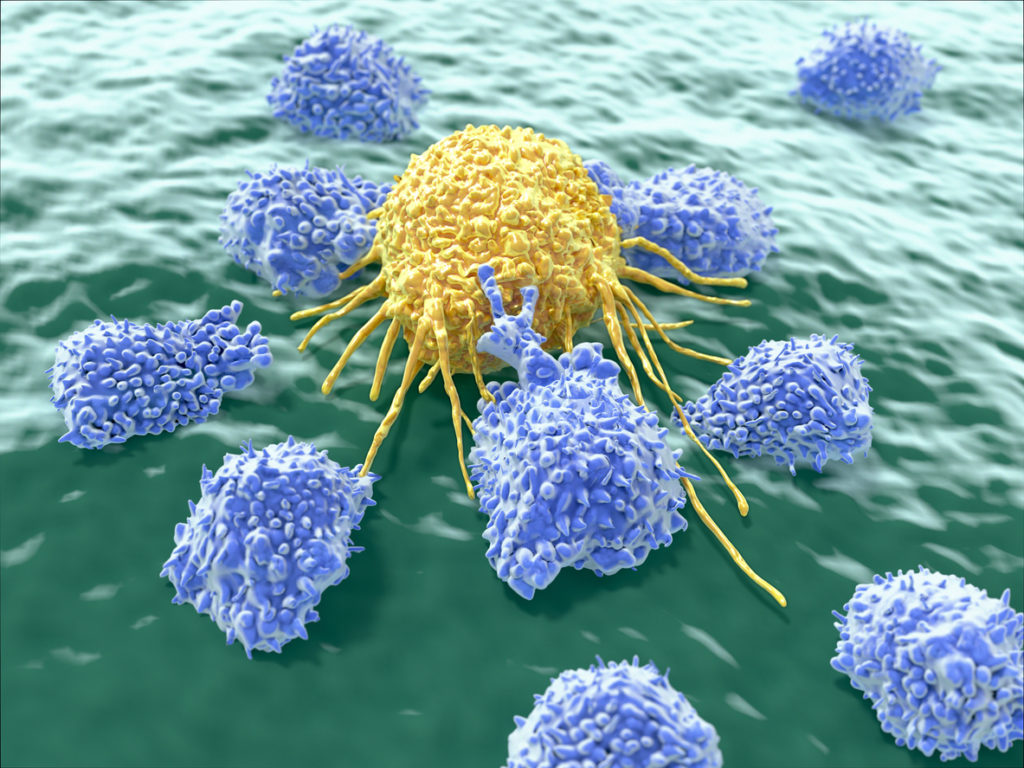
Natural Killer (NK) cells
Natural killer (NK) cells are a type of lymphocyte (white blood cell) that are a critical component of the innate immune system. They play a major role in the destruction of both cancer cells and virus-infected cells, as well as in the maintenance of self-tolerance. Individuals with high levels of NK cells may not develop full-blown disease even if the virus penetrates their cells, as NK cells will spring into action killing viral-infected cells and tumor cells. Therefore, people with low mucus, low secretory IgA and low NK cell activity are the most prone to develop disease. Individuals with high NK cell activity will prevent replication and the spread of the virus. In people with low mucus, low secretory IgA and low natural killer cell activity, the virus WILL spread, leading to the next stage of disease.
Inflammatory Pathways Upregulated
Recent research is showing that the SARS-COV2 virus upregulates inflammatory pathways in the human body which allows it to proliferate. SARS-COV2 uses pre-existing pro-inflammatory pathways to initiate an increased inflammatory response which can include, in more serious cases, immune hyper-reactivity known as ‘cytokine storm’.
Alterations in the immune system that result in chronic inflammation and autoimmunity have been linked to a wide range of health conditions and diseases. Cytokines are important chemical mediators and signalling molecules of both innate and adaptive immune responses, and imbalances in cytokines have been shown to play key roles in chronic inflammation and autoimmunity. Most cytokines can be generally characterized as pro-inflammatory or anti-inflammatory, depending on their primary effects on immune responses. Immune responses are often further characterized by the type of T helper (Th) cell involved.
Depending on the inflammatory response taking place (ie: Th1, Th2, Th17, T-reg, etc.), different cytokines will be produced that mediate a specific inflammatory or anti-inflammatory pathway. Clinicians can gain insight into the inflammatory pathways that are upregulated in patients with immune disorders, such as those commonly seen in chronic inflammation and autoimmune disease by assessing levels of cytokines and identifying patterns of cytokine activity.
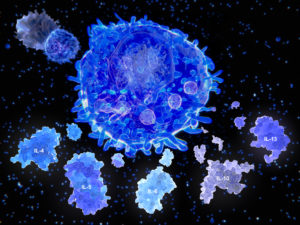
A certain amount of cytokine activity is normal in infections or even in non-infected individuals, but excessive cytokine activity, especially in the presence of the SARS-COV2 virus, can be detrimental as it causes damage to lung tissue and leads to cell destruction. The increased lung cell destruction allows debris and fluids to build up in the lungs affecting oxygen exchange, leading to cough, tightness of chest, pneumonia and difficulty breathing. As it progresses, it can lead to a surge of cytokine activity known as ‘cytokine storm’ and acute respiratory distress syndrome (ARDS). We will explore this topic of ARDS, including why this virus leads to ARDS in some people but not in others, in more depth in the next article in this series and what you can do to prevent this from happening.
What can you do to protect yourself?
 Identify Compromised Mucosal Immunity and Immune Tolerance
Identify Compromised Mucosal Immunity and Immune Tolerance
Cyrex Array 14 Mucosal Immune Reactivity Screen
 Cyrex Array 14 Mucosal Immune Reactivity Screen can help identify compromised immune tolerance and mucosal immunity, intestinal barrier dysfunction, food, chemical and pathogen reactivity, and blood brain barrier integrity all from a simple saliva sample. Can be done from the convenience of your home with a simple saliva sample. Kits can be drop-shipped. Cost is $339.
Cyrex Array 14 Mucosal Immune Reactivity Screen can help identify compromised immune tolerance and mucosal immunity, intestinal barrier dysfunction, food, chemical and pathogen reactivity, and blood brain barrier integrity all from a simple saliva sample. Can be done from the convenience of your home with a simple saliva sample. Kits can be drop-shipped. Cost is $339.
Click here for more info on Cyrex Array 14 Mucosal Immune Reactivity Screen
 Adrenal Stress Index Profile (ASI)
Adrenal Stress Index Profile (ASI)
What tests are included in the ASI Panel?
- Cortisol–Evaluates stress response over a 24-hour period to establish the cortisol circadian rhythm
- DHEA/DHEA-S–Very important precursor to many steroid hormones, including sex hormones and determines how these may be affected by stress
- Total secretory IgA (sIgA)–Evaluates the toll of stress on mucosal immunity, a critical part of immune health
- 17-OH Progesterone–Helps determine underlying causes of abnormal cortisol levels
- Insulin–Investigates blood sugar control and insulin resistance
- Wheat gluten sIgA–Identifies immune response to gluten (gluten sensitivity)
Can be done from the convenience of your home with a simple saliva sample. Kits can be drop-shipped. Cost is $125.
Click here for more info on the Adrenal Stress Index Profile
 Cytokine Response Profile
Cytokine Response Profile
CytoDx® Panel Enables Practitioners to Assess Systemic Immune Status:
- Pro-inflammatory / anti-inflammatory cytokine balance
- Th1, Th2, Th17 and T-regulatory cytokine responses
- Efficacy of immune balancing and modulating approaches
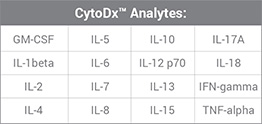 The CytoDx panel assesses levels of all the major cytokines involved in pro-inflammatory and anti-inflammatory reactions. It requires a simple blood draw. Kits can be drop-shipped. Cost is $295.
The CytoDx panel assesses levels of all the major cytokines involved in pro-inflammatory and anti-inflammatory reactions. It requires a simple blood draw. Kits can be drop-shipped. Cost is $295.