 In this issue, we discuss the strategies used to manage autoimmune disease in functional medicine, including dietary and lifestyle strategies and the use of key supplements targeting the major areas of immune dysfunction and autoimmune reactions as identified by the most current literature and described in part 6.
In this issue, we discuss the strategies used to manage autoimmune disease in functional medicine, including dietary and lifestyle strategies and the use of key supplements targeting the major areas of immune dysfunction and autoimmune reactions as identified by the most current literature and described in part 6.
 Management of Autoimmune Disease in Functional Medicine: A Different Approach to Autoimmunity
Management of Autoimmune Disease in Functional Medicine: A Different Approach to Autoimmunity
When you look at the dysregulation of the immune system that occurs in autoimmunity, we know there are several systems involved in the targeting of self-tissue for destruction. The goal of autoimmune management should be to dampen or decrease the immune attacks against self-tissue. In order to do this, we have to understand these various systems that become dysregulated and ultimately lead to autoimmunity and self-tissue destruction. In functional medicine, we attempt to modulate autoimmunity by regulating these various systems with the use of natural compounds that have been identified in the literature to have an impact on these mechanisms of immune dysfunction. In part 6 of this series, we identified several key mechanisms of immune dysfunction which have been shown to play a role in the development of autoimmune reactivity and autoimmunity. Let’s briefly review these mechanisms and their role in autoimmunity and then look at the evidence in the literature supporting the use of various natural compounds in regulating each of these mechanisms. This information is a bit technical, so for those that are not interested in the following technical information but are interested in learning about how dietary and lifestyle changes can improve autoimmune expression and the progression of autoimmune disease, please skip down to the final sections of this article, Lifestyle Strategies for Autoimmunity and Dietary Strategies for Autoimmunity.
For a more thorough review of these various mechanisms and how they are involved in autoimmunity, please refer to part 6 of this series.
 Major Mechanisms of Immune Dysfunction in Autoimmunity
Major Mechanisms of Immune Dysfunction in Autoimmunity
- NF-kB activation and dampening
- Glutathione recycling system
- Barrier system integrity
- TH-1 and TH-2 polar shifts
- TH-3 or regulatory T-cell activation
- TH-17 activation and dampening
- Nitric oxide isomer expressions
 NF-kB Activation
NF-kB Activation
In the last segment of this series, we learned that an increasing number of studies indicate that a protein complex known as NF-kB plays an important role in the development and progression of autoimmunity. [1] NF-kB controls the expression of genes encoding the proinflammatory mediators involved in autoimmune disease. NF-kB can become chronically elevated because of its own amplifying loop. [2] This means that elevations in NF-kB can perpetuate further elevations of NF-kB. NF-kB signaling needs to be downregulated in order to maintain tissue homeostasis. [3] If this does not occur, then immune dysregulation takes place that can eventually lead to autoimmunity. Recent data has shown that NF-kB is required for activation of immune cells against self-tissue, and its hyperactivity should be minimized, since it promotes autoimmunity. [4]
“NF-kB is a key signaling component in autoimmunity and an attractive target for autoimmune disease therapy” [5]
“Since NF-kB represents an important and very attractive therapeutic target for drugs to treat many inflammatory diseases, including arthritis, asthma and the autoimmune diseases, most attention has been paid in the last decade to the identification of compounds that selectively interfere with this pathway” [6]
Clinical Strategies to Dampen NF-kB Activation
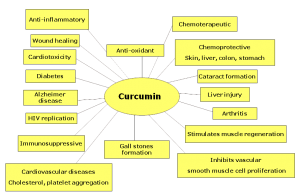
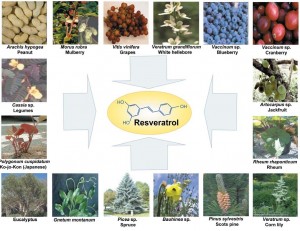
Overall, it appears that the two most potent natural NF-kB minimizers that researchers have discovered are curcumin and resveratrol. Curcumin is the alkaloid derived from turmeric, a spice used in Indian cooking. Resveratrol is a compound found in the skin of red grapes, peanuts and some berries. In recent studies, both curcumin and resveratrol have shown to support healthy numbers of T-cell cytokines (inflammatory messengers). These results suggest the potential use of these select phytochemicals for supporting healthy immune responses. Here is what some recent literature is saying about these compounds:
 Curcumin
Curcumin
“Curcumin, a dietary spice from turmeric, has outstanding anti-inflammation and neuroprotective effects. Herein, we review key features of curcumin involved in biology, pharmacology, and medicinal chemistry and discuss its potential relevance to pathophysiological progress of multiple sclerosis”[7]
“Curcumin, a component of turmeric, has been shown to be non-toxic, to have antioxidant activity, and to inhibit such mediators of inflammation as NFkB, cyclooxygenase-2 (COX-2), lipooxygenase (LOX), and inducible nitric oxide synthase (iNOS)”[8]
“In a larger, randomized, double-blind, multicenter trial involving patients with quiescent ulcerative colitis, administration of 1 g of curcumin twice daily resulted in both clinical improvement and a statistically significant decrease in the rate of relapse”[9]
 “Our results indicate that curcumin has the potential to protect against cardiac inflammation… and may provide a novel therapeutic strategy for autoimmune myocarditis” [10]
“Our results indicate that curcumin has the potential to protect against cardiac inflammation… and may provide a novel therapeutic strategy for autoimmune myocarditis” [10]
“In various chronic illnesses in which inflammation is known to play a major role, curcumin has been shown to exhibit therapeutic potential. These diseases include Alzheimer’s disease (AD), Parkinson’s disease, multiple sclerosis, epilepsy, cerebral injury, CVDs, cancer, allergy, asthma, bronchitis, colitis, rheumatoid arthritis, renal ischemia, psoriasis, diabetes, obesity, depression, fatigue, and AIDS”[11]
“…curcumin has received considerable interest as a potential therapeutic agent for the prevention and/or treatment of various malignant diseases, arthritis, allergies, Alzheimer’s disease, and other inflammatory illnesses. The underlying mechanisms of these effects are diverse and appear to involve the regulation of various molecular targets, including transcription factors (such as nuclear factor-kB)…”[12]
 Resveratrol
Resveratrol
Resveratrol has been shown in the literature to have powerful anti-inflammatory effects which involve multiple pathways including inhibiting NF-kB, inhibiting iNOS (inducible-nitric oxide synthase) expression and inhibiting inflammatory cytokines such as IL-6 in various inflammatory conditions, including multiple sclerosis [13], diabetic neuropathy [14], arthritis [15], autoimmune myocarditis [16], colitis [17], and exerts immunomodu-latory effects both in vitro and in vivo lymphocytic leukemia in lymphocytic leukemia. [18]
“These studies demonstrate that SRT501 (a pharmaceutical grade formulation of resveratrol) attenuates neuronal damage and neurological dysfunction in experimental autoimmune encephalomyelitis (the animal model of multiple sclerosis) by a mechanism involving SIRT1 activation”[13]
 “This study confirms the NF-kB inhibitory activity and anti-inflammatory activity of resveratrol, which may contribute to neuroprotection in diabetic neuropathy apart from its antioxidant effect”[14]
“This study confirms the NF-kB inhibitory activity and anti-inflammatory activity of resveratrol, which may contribute to neuroprotection in diabetic neuropathy apart from its antioxidant effect”[14]
“In summary, our results suggest that resveratrol suppresses apoptosis and inflammatory signaling through its actions on the NF-kB pathway in human chondrocytes”[15]
“Resveratrol significantly ameliorated myocardial injury and preserved cardiac function in a rat model of autoimmune myocarditis” [16]
In addition, both curcumin and resveratrol have been shown to reduce the inflammatory mediators that contribute to the low-level, chronic inflammation found in obese individuals and have been linked to the onset of cardiovascular disorders, insulin resistance and type 2 diabetes mellitus.
“Curcumin and resveratrol are able to inhibit TNFalpha-activated NF-kappaB signaling in adipocytes and as a result significantly reduce cytokine expression. These data suggest that curcumin and resveratrol may provide a novel and safe approach to reduce or inhibit the chronic inflammatory properties of adipose tissue.”[19]
 The Role of Glutathione in Autoimmune Modulation
The Role of Glutathione in Autoimmune Modulation
In part 6, we learned that glutathione (GSH) appears to be an important antioxidant that helps support regulatory TH-3 cells of the immune system (important immune-regulating cells), protect the intestinal barrier and quench oxidative compounds before they are activated by the NF-kB receptor. Recall that glutathione production occurs in all cells but primarily in hepatocytes (the cells of the liver) which is used in some phase II detox pathways, such as glutathione conjugation. Glutathione is also an important part of phase I detoxification. Some medications (such as acetaminophen or Tylenol™) block the production of glutathione by the liver. This is why glutathione precursors (such as N-acetyl-cysteine or NAC) is used to decrease liver toxicity in acetaminophen overdose. [20] Many studies suggest that intracellular GSH levels in antigen-presenting cells such as macrophages, influence the TH1/TH2 cytokine response patterns which are associated with immune dysregulation and autoimmunity.
“Accumulation of evidence suggests that intracellular GSH levels in antigen-presenting cells such as macrophages, influence the TH1/TH2 cytokine response pattern. The observations reported herein show that pro-GSH molecules represent new therapeutic agents to support immune modulation”[21]
 Low GSH levels have been associated with many autoimmune and inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease, multiple sclerosis, psoriasis, and contact dermatitis. [22]
Low GSH levels have been associated with many autoimmune and inflammatory diseases, including rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease, multiple sclerosis, psoriasis, and contact dermatitis. [22]
Another study suggested that the severity of some autoimmune diseases, such as systemic lupus erythematosus (SLE), correlate with levels of glutathione in the blood, namely, the lower the glutathione levels, the more severe the autoimmune disease.
“A significant correlation between plasma glutathione and SLE severity exists that may aid evaluation of the disease severity and usefulness of the management of SLE”[23]
 In general, high oxidant burdens are a common feature in many immune dysfunctions and GSH plays a major role in quenching these oxidant species, and hence protecting the cell from damage. Therefore, any decrease in GSH may influence the immune system through perpetuating reactive species signaling events and increasing ROS-related damage. [24]
In general, high oxidant burdens are a common feature in many immune dysfunctions and GSH plays a major role in quenching these oxidant species, and hence protecting the cell from damage. Therefore, any decrease in GSH may influence the immune system through perpetuating reactive species signaling events and increasing ROS-related damage. [24]
Age-related decline in cellular function is thought to result from increasing oxidative damage. Oxidative stress depletes intracellular GSH, so increasing production of glutathione may protect against mitochondrial and cellular damage. [25]
Glutathione helps support regulatory T cells, and differentiation of T-cells into their specific types by reducing damage from oxidative stress. This system is absolutely critical for overall modulation of autoimmunity.
“These data indicate that glutathione peroxidase-dependent control of intracellular reactive oxygen species accumulation is important not only for regulation of TH-cell proliferation, but also for modulation of differentiation into TH1, TH2 and TH17 cells.”[26]
Summary of the Role of Glutathione in Autoimmune Modulation
- Modulates TH-1 and TH-2 polarization which dampens AI tissue destruction
- Activates TH-3 regulatory T-cells which dampens AI tissue destruction
- Dampens TH-17 activity which dampens AI tissue destruction
- Enhances tissue and intestinal regeneration which enhances AI recovery
- Protects cell mitochondria which enhances AI recovery
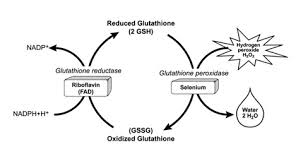 Clinical Strategies to Support Glutathione Levels and Glutathione Recycling
Clinical Strategies to Support Glutathione Levels and Glutathione Recycling
If you look at the literature, there are several natural compounds that have been shown to help raise intracellular glutathione and support the glutathione recycling system:
- N-acetyl-cysteine
- Alpha lipoic acid
- L-glutamine
- Selenium
- Cordyceps
- Centella asiatica
- Silybum marianum
- Broccoli extract
 N-Acetyl-Cysteine (NAC)
N-Acetyl-Cysteine (NAC)
N-acetyl-cysteine is a metabolite of the sulfur-containing amino acid, cysteine. It plays a role in the sulfation cycle, acting as a sulfur donor in phase II detoxification and as a methyl donor in the conversion of homocysteine to methionine. N-acetyl cysteine is rapidly metabolized to intracellular glutathione. It is used for acetaminophen overdose and as a nephroprotective agent for radiocontrast. [27-33]
 Alpha lipoic acid
Alpha lipoic acid
Alpha lipoic acid is an antioxidant that is made by the body and is found in every cell, where it helps turn glucose into energy. Unlike other antioxidants, which work only in water (such as vitamin C) or fatty tissues (such as vitamin E), alpha lipoic acid is both fat- and water-soluble. This means it can work throughout the body. Alpha lipoic acid also plays an important role in the synergism of antioxidants. It directly recycles and extends the metabolic life spans of vitamin C, glutathione, and coenzyme Q10, and it indirectly renews vitamin E, all of which are necessary for glutathione recycling. [34-43]
 L-Glutamine
L-Glutamine
L-glutamine is important for the generation of glutathione stores since glutamate is unable to be transported into cells. Glutamine is efficiently transported into the cell, converted to glutamate, and readily available for glutathione synthesis. Research has demonstrated that glutamine is important for the generation of glutathione. [44-57]
 Selenium
Selenium
Selenium is a trace element nutrient that serves as the essential cofactor for the enzyme glutathione peroxidase. Selenium-deficient humans and animals are known to be deficient in glutathione peroxidase activity in their cells and plasma. [58-69]
 Cordyceps
Cordyceps
Cordyceps (used in Chinese medicine for thousands of years) has been shown to activate glutathione peroxidase synthesis in the body (the enzymes that increase glutathione) and raise glutathione levels within minutes. Research has demonstrated cordyceps helps protect cells by engaging the glutathione enzyme cycle. [70-73]
 Centella asiatica (Gotu Kola)
Centella asiatica (Gotu Kola)
Research has clearly demonstrated that oral intake of gotu kola very rapidly and dramatically increases the activity and amount of glutathione peroxidase and quantity of glutathione. [74-84]
 Silybum marianum
Silybum marianum
Administration of Silybum marianum has shown to significantly increase glutathione, increase superoxide dismutase activity, and have positive influence in the ratios of reduced and oxidized glutathione. [85-101]
 Broccoli extract
Broccoli extract
Broccoli has a high content of glucosinolates, which are metabolized into isothiocyanates and sulforaphane [102]. These compounds exhibit chemoprotective activity through a mechanism involving inhibition of cytochrome P450 and induction of GST and other enzymes [103,104].
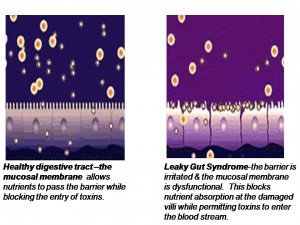 Barrier System Integrity
Barrier System Integrity
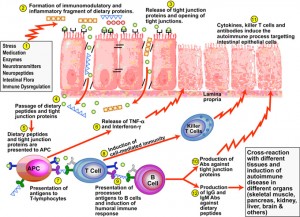
In part 6, we learned when the intestinal mucosa of the digestive tract is inflamed, the tight junctions of the intestinal mucosa are compromised and they become widened and permeable to large, undigested compounds, toxins and bacteria which can then enter the bloodstream. This condition is known as leaky gut. These large undigested proteins that are absorbed are reacted against by the underlying intestinal immune system and promote exaggerated immune responsiveness (inflammation) which leads to further destruction of the intestinal barrier. This creates a vicious cycle of further intestinal inflammation and greater loss of intestinal integrity. As the intestinal tract becomes inflamed from diet, lifestyle, medications, and infections, it promotes further intestinal inflammation and further intestinal permeability. Most importantly, the immune dysregulation from increased permeability has been shown to perpetuate immune overzealousness and loss of ability to regulate self and non-self tolerance, leading to autoimmunity.
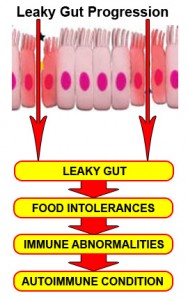 “A more attentive analysis of the anatomic and functional arrangement of the GI tract however suggests that another extremely important function of this organ is its ability to regulate the trafficking of macromolecules between the environment and the host through a barrier mechanism. Together with the gut-associated lymphoid tissue and the neuroendocrine network, the intestinal epithelial barrier, with its intercellular tight junctions, controls the equilibrium between tolerance and immunity to nonself-antigens. When the finely-tuned trafficking of macromolecules is dysregulated in genetically susceptible individuals, both intestinal and extraintestinal autoimmune disorders can occur.”[105]
“A more attentive analysis of the anatomic and functional arrangement of the GI tract however suggests that another extremely important function of this organ is its ability to regulate the trafficking of macromolecules between the environment and the host through a barrier mechanism. Together with the gut-associated lymphoid tissue and the neuroendocrine network, the intestinal epithelial barrier, with its intercellular tight junctions, controls the equilibrium between tolerance and immunity to nonself-antigens. When the finely-tuned trafficking of macromolecules is dysregulated in genetically susceptible individuals, both intestinal and extraintestinal autoimmune disorders can occur.”[105]
“There is growing evidence that increased intestinal permeability plays a pathogenic role in various autoimmune diseases. Therefore, we hypothesize that loss of intestinal barrier function is necessary to develop autoimmunity.”[106]
Clinical Strategies to Support the Barrier System
 L-Glutamine
L-Glutamine
L-glutamine is the preferred fuel source for the cells of the small intestine and has been shown in numerous studies to support the regeneration and repair of the intestinal lining. It has also been shown to increase the number of cells in the small intestine, the number of villi on those cells, as well as the height of the villi. Glutamine-reduced permeability of the lining may accompany “leaky gut” patterns that promote intestinal inflammation and the development of delayed food intolerances. [107-128]
 Deglycyrrhizinated Licorice
Deglycyrrhizinated Licorice
Deglycyrrhizinated licorice is a popular and substantially studied natural compound that provides flavonoids that help heal the gastric and intestinal lining. Many different mechanisms have been shown with regard to its restorative properties including stimulation and differentiation of glandulars cells, protective mucous formation, protective mucous secretion, increased intestinal blood flow, and growth and regeneration of intestinal lining cells. [129-147]
 N-Acetyl Glucosamine
N-Acetyl Glucosamine
N-acetyl glucosamine is a monosaccharide derivative of glucose that is used to support the intestinal glycoprotein cover of the mucosa called mucin. It is also a precursor substrate for the repair of the intestinal mucosal cells and provides support of mucosa membrane irritation. [148-151]
 Aloe Leaf Extract
Aloe Leaf Extract
Aloe leaf extract contains natural phytochemicals and powerful antioxidant properties that reduce intestinal inflammation, soothe the intestines, aid in intestinal wound healing, and have an anti-ulcer effect. It also appears to have antifungal properties, supports cholinergic intestinal motility, and reduces intestinal pain and discomfort. [152-161]
 Spanish Moss
Spanish Moss
Spanish moss is also known as Tillandsia and it has historically been used for intestinal irritation and allergies. Research on the plant has identified rich sources of flavonoids and other phytochemicals that provide antimicrobial activity and free radical scavenging properties. [162-167]
 Marshmallow Extract
Marshmallow Extract
Marshmallow extract has high content of mucilage that can soothe and help heal compromised intestinal barrier tissue. It is also rich in antioxidants that can support healing of tissue. It also has properties that inhibit hyaluronidase, which is the enzyme involved in the production of hyaluronic acid that is involved with intestinal tissue destruction. [168-171]
 Methylsulfonylmethane (MSM)
Methylsulfonylmethane (MSM)
Methylsulfonylmethane (MSM) is a rich source of natural sulfur which helps as a substrate for antioxidant defense systems as well as support substrates for hepatic phase II sulfation pathways. It has antifungal and anti-inflammatory properties that help support the compromised liver-gut axis. [172-176]
 Gamma Oryzanol
Gamma Oryzanol
Gamma oryzanol is a mixture of plant sterols and ferulic acid esters from rice. It has demonstrated to be a powerful antioxidant. Numerous papers have demonstrated its effectiveness in gastrointestinal complaints, ulcers, irritable bowel syndrome and non-specific gastrointestinal conditions. It has also been shown to modulate and support the enteric nervous system in its ability to activate intestinal motility and secrete digestive enzymes. [177-184]
 Slippery Elm Bark
Slippery Elm Bark
Slippery elm bark is very high in natural mucilage and helpful in soothing the inflamed intestinal cells. It reduces contact of inflammatory proteins with the intestinal mucosa, thereby enhancing recovery from intestinal barrier compromise and inflammation. [185-188]
 German Chamomile
German Chamomile
The chief constituents of German chamomile have been shown to enhance wound-healing time, modulate prostaglandins and nitric oxide activity to provide gastric and intestinal protection. [189-196]
 Marigold Flower Extract
Marigold Flower Extract
Marigold flower extract constituents include saponins, carotenoids, flavonoids, mucilage, bitter principle, phytosterols, polysaccharides, and resin. It has been used historically for varied gastrointestinal complaints. It provides substrates for digestive enzyme production, helps during inflammation and provides antibacterial activity. [197-204]
 Glutathione
Glutathione
When a person loses their ability to recycle glutathione and glutathione levels become depleted, they are at risk for intestinal lining inflammation which then leads to leaky gut. The glutathione recycling system is one of the main systems that prevents leaky gut onset. Glutathione is suggested to play an important role in gut barrier function and prevention of intestinal inflammation. [205-210]
 Zinc Carnosine
Zinc Carnosine
“Regarding intestinal permeability, zinc carnosine caused an approximate threefold increase in gut integrity and repair”[211]
Vitamin D
“VDR (vitamin D receptor) plays a critical role in mucosal barrier homeostasis by preserving the integrity of junction complexes and the healing capacity of the colonic epithelium. Therefore, vitamin D deficiency may compromise the mucosal barrier, leading to increased susceptibility to mucosal damage and increased risk of IBD (inflammatory bowel disease).” [212]
 “There is growing appreciation of the importance of the pleiotropic hormone vitamin D in the development of tolerance, immune system defenses, and epithelial barrier integrity” [213]
“There is growing appreciation of the importance of the pleiotropic hormone vitamin D in the development of tolerance, immune system defenses, and epithelial barrier integrity” [213]
“1,25(OH)2D3 (vitamin D) may play a protective role in mucosal barrier homeostasis by maintaining the integrity of junction complexes and in healing capacity of the colon epithelium. 1,25(OH)2D3 may represent an attractive and novel therapeutic agent for the adjuvant therapy of IBD (inflammatory bowel disease).”[214]
Intestinal Permeability Dietary Restrictions
It is important to avoid certain types of food that tend to aggravate or worsen intestinal permeability when trying to repair the gut barrier and decrease autoimmune responses. These self-promoting vicious cycles become difficult to unwind unless aggressive dietary and nutritional strategies are employed. See Intestinal Permeability Dietary Restrictions at the end of this article for a complete list of these foods.
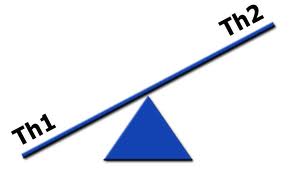 TH-1 and TH-2 Polar Shifts
TH-1 and TH-2 Polar Shifts
As we learned in part 6, the immune system has two major immune responses:
- T-helper-1 (TH-1) response, which is mediated by T-cells
- T-helper-2 (TH-2) response, which is mediated by B-cells
Both immune responses are important for protecting the body against foreign microbes and invaders. There is a constant interplay and subtle balance of these two immune responses and the regulation of these two responses is extremely important. This is carried out by the TH-3 regulatory system (described below), which acts in many ways like the conductor of a large complex orchestra. In healthy immune responses, there is always a balance in these two systems. In autoimmunity, there is a polar shift or dominance of either the TH-1 or TH-2 system and one side of the system becomes overactive or dominant. Many autoimmune diseases are classified in the immunology literature by their T-helper dominance.[215, 216] Let’s review the TH-3 regulatory system.
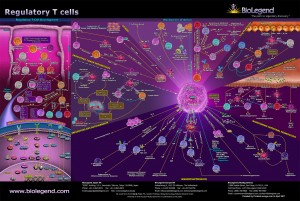 Regulatory T-Cell (TH-3 Cell) Activation
Regulatory T-Cell (TH-3 Cell) Activation
As discussed in part 6, TH-3 cells, or regulatory T-cells, are a subpopulation of T-cells that downregulate the immune system, maintain tolerance to self-antigens and minimize autoimmune disease. Regulatory T-cells are a component of the immune system that suppress immune responses from other cells. This is an important “self-check” mechanism inherent in the immune system to prevent excessive reactions. These cells are involved in shutting down the immune responses after they have successfully eliminated invading organisms and also in preventing autoimmune TH-1 and TH-2 polar shifts. [217, 218]
 Clinical Strategies to Support Regulatory T-Cell Activation and Balance TH-1 and TH-2 Polar Shifts
Clinical Strategies to Support Regulatory T-Cell Activation and Balance TH-1 and TH-2 Polar Shifts
Two key nutrients have been shown over and over in the literature to support the regulatory T-cells and coordinate TH-1 and TH-2 balance: glutathione and vitamin D.
 Glutathione
Glutathione
Glutathione (GSH) is one of the most critical immune-modulating substances that we know of. Like a conductor of an orchestra, glutathione regulates various aspects of immune balance. As previously discussed, studies show that GSH has a significant impact on the immune system’s ability to activate the appropriate T-helper cell response. [219-220] Because GSH is so important in the immune system’s activation of the appropriate T-helper response, altering its levels may have significant implications in TH1/TH2-related diseases. [221]
“Accumulation of evidence suggests that intracellular GSH (glutathione) levels in antigen-presenting cells such as macrophages, influence the TH1/TH2 cytokine response pattern. The observations reported herein show that pro-GSH molecules represent new therapeutic agents to support immune modulation.” [222]
“These data indicate that glutathione peroxidase-dependent control of intracellular reactive oxygen species accumulation is important not only for regulation of TH-cell proliferation, but also for modulation of differentiation into TH1, TH2 and TH17 cells.”[223]
See the clinical strategies described above for strategies for increasing glutathione levels.
 Vitamin D
Vitamin D
Numerous studies have been published establishing the critical immunoregulatory role that vitamin D plays in the prevention of immune dysfunction and the development of autoimmune disease. [224-231] Vitamin D appears to act on a number of different immune cells to promote various regulatory responses by the immune system. Vitamin D deficiency has been associated with many autoimmune disorders including multiple sclerosis, Type 1 diabetes, Crohn’s disease, rheumatoid arthritis as well as others. [232]
Studies have shown a significant relation between vitamin D deficiency and allergy and an important role of vitamin D in the pathogenesis and severity of allergic disease, and its capacity to control allergic disease. [233, 234] Vitamin D supplementation has been shown to reduce the occurrence of asthma in most related studies and may be useful in the prevention or adjunct treatment of chronic obstructive pulmonary disease. [235, 236]
Here are a few selections from the literature on the role vitamin D plays in immune health:
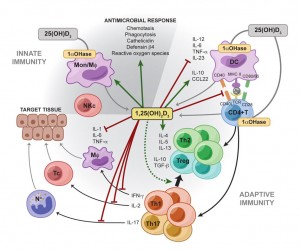 “Increasing evidence demonstrates a strong association between vitamin D signaling and many biological processes that regulate immune responses. The discovery of the vitamin D receptor in multiple immune cell lineages, such as monocytes, dendritic cells, and activated T cells credits vitamin D with a novel role in modulating immunological functions and its subsequent role in the development or prevention of autoimmune diseases.”[237]
“Increasing evidence demonstrates a strong association between vitamin D signaling and many biological processes that regulate immune responses. The discovery of the vitamin D receptor in multiple immune cell lineages, such as monocytes, dendritic cells, and activated T cells credits vitamin D with a novel role in modulating immunological functions and its subsequent role in the development or prevention of autoimmune diseases.”[237]
“It is apparent that vitamin D has significant effects on the immune system and as such may contribute to the pathogenesis of autoimmune disease. Low vitamin D status is reported in many inflammatory rheumatic conditions. In some this extends to an association with disease activity. Vitamin D acts on a number of cells involved in both innate and acquired immunity biasing the adaptive immune system away from Th17 and Th1, towards Th2 and T-regs (T3). Deficiency accordingly could encourage autoimmunity. Vitamin D deficiency may well be an important factor in autoimmune rheumatic disease, including initial disease development and worsening the disease once present.”[238]
 TH-17 Activation and Dampening
TH-17 Activation and Dampening
As discussed in part 6, T-helper 17 (TH-17) is a subset of T-helper cells producing IL-17. IL-17 is a potent proinflammatory cytokine that amplifies ongoing inflammation in epithelial and endothelial cells. [239-243] TH-17 is important for normal immunity, but it can also become excessively active, especially in those with autoimmunity. TH-17 activity is considered a key marker in the activation and severity of autoimmune flare-ups. TH-17 responses stimulate autoimmunity and TH-17 activation is like “adding fuel to the fire” of autoimmunity. The inhibition or attenuation of TH-17 cells is now considered a therapeutic strategy for the management of autoimmune disease in the literature:
“The absence of Th17 cells can lead to a decrease in proinflammatory cytokines. Thus, to achieve the end goal of a balanced immune response, CAM (Complementary and Alternative Medicine) practitioners may target components along the pathway leading from naïve T-cell differentiation to Th17, thereby lowering the levels of Th17 and IL-17.” [244]
Clinical Strategies to Dampen TH-17 Activation
There are two natural compounds which have shown to inhibit or minimize TH-17 activation in the literature: curcumin and resveratrol.
 Curcumin
Curcumin
There is substantial evidence that curcumin minimizes the activity of TH-17. [8-12] A recent paper entitled, “Curcumin has bright prospects for the treatment of multiple sclerosis,” demonstrated that curcumin supports healthy anti-inflammatory and neurosupportive mechanisms. The researchers concluded that TH-17 cells are key immunological players for the pathophysiological process of multiple sclerosis and curcumin has outstanding effects in dampening this TH-17 system.
“Th17 cells are considered as a key immunological player for the pathophysiological process of MS. Curcumin, a dietary spice from turmeric, has outstanding anti-inflammation and neuroprotective effects. Herein, we review key features of curcumin involved in biology, pharmacology, and medicinal chemistry and discuss its potential relevance to pathophysiological progress of MS”[245]
Another study found that providing curcumin supplementation to Lewis rats significantly reduced the clinical severity of experimental autoimmune encephalomyelitis (EAE), and had a dramatic reduction in the number of inflammatory cells that infiltrated the spinal cord by dampening the TH-17 system.
“These findings indicated that curcumin amelioration EAE was, to a large extent, due to inhibiting differentiation and development of Th17 cells…and suggest it is useful in the treatment of MS and other Th17 cell-mediated inflammatory diseases.” [246]
 Resveratrol
Resveratrol
Resveratrol appears to modulate immunological processes via multiple mechanisms. As we saw above, resveratrol has potent anti-inflammatory effects and has been shown to dampen inflammatory mediators including NF-kB, iNOS (inducible nitric oxide synthase) and various inflammatory cytokines. Several papers have also been published on the role of resveratrol and its ability to modulate TH-17 differentiation and activity as a potential complementary therapy for neuroautoimmune disease such as multiple sclerosis. [247-248] In fact, activation of NF-kB and other inflammatory cytokines has been associated with TH-17 activation and resulting neuroinflammatory processes and tissue destruction.
“Therefore, the modulation of NF-κB and associated cytokines such as IL-1β, IL6, IL-8 and TNF-α by using one or a combination of different herbal medicines may suppress Th17 and IL-17 production and inhibit neuroinflammation. CAM practitioners may thus consider NF-κB modulation for therapeutic intervention or maintenance of neuroimmune inflammatory disorders.”[248]
 Research has pointed to transcription factor aryl hydrocarbon receptor (AHR) as a regulator of T-regulatory cells (T-reg cells) and TH17 cell differentiation. [249]
Research has pointed to transcription factor aryl hydrocarbon receptor (AHR) as a regulator of T-regulatory cells (T-reg cells) and TH17 cell differentiation. [249]
“Thus, AHR regulates both T(reg) and T(H)17 cell differentiation in a ligand-specific fashion, constituting a unique target for therapeutic immunomodulation.” [250]
Resveratrol is a natural AHR antagonist. This is one of the mechanisms by which resveratrol is believed to decrease the activity of Th17 cells and has become an attractive therapeutic option for autoimmune disease.
“Resveratrol, a polyphenolic compound derived from grapes, suppressed swelling and bone erosion in the paws of mice with CIA (collagen-induced arthritis). This effect was associated with reduced serum levels of proinflammatory cytokines including IL-17 and reduced numbers of Th17 cells”[251]
Therefore, it appears that both curcumin and resveratrol impact two major inflammatory pathways in our current understanding of autoimmune physiology: TH-17 activation and NF-kB activation.
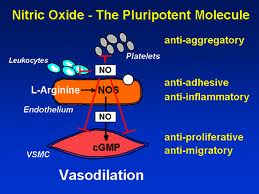 Nitric Oxide Isomer Expressions
Nitric Oxide Isomer Expressions
As we previously learned, nitric oxide (NO) is an important messenger molecule involved in many physiological and pathological processes as well as a chemical-signalling molecule that is involved with communication in the nervous system, immune system and vascular system. It was proclaimed the “Molecule of the Year” in 1992. [252]
Nitic oxide plays both destructive and protective roles in autoimmunity, depending on which of the three enzymes, or synthases, is expressed. The three nitric oxide synthase (NOS) isomers (forms) are neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS). nNOS supports nervous tissue, including the brain, and neurological synapses involved in neuronal signaling and brain function. eNOS activity supports blood vessel health by producing dilation of blood vessels, dissolves endothelial plaques, and enhances blood flow. [253, 254] iNOS, however, has destructive effects on tissue. iNOS is activated by TH-17 and produces harmful free radical action and initiates tissue destruction. [254] How these isomers are manipulated or modulated will determine how much destruction of tissue or ability to recover from autoimmune flare-ups a person has.
 Studies show that nitric oxide (NO) modulates inflammatory and immune responses in multiple ways and plays a protective role in many forms of inflammatory and autoimmune reactions. [255-258]
Studies show that nitric oxide (NO) modulates inflammatory and immune responses in multiple ways and plays a protective role in many forms of inflammatory and autoimmune reactions. [255-258]
“We conclude that NO (nitric oxide) plays a critical immunoregulatory role and NO modulation may prevent the onset of autoimmune reactions”[259]
Research has shown that IL-17 and the TH-17 system activates iNOS and initiates tissue destruction in autoimmunity:
“Interleukin-17 is a proinflammatory cytokine involved in autoimmune disorders. The proinflammatory action of IL-17 depends considerably on its ability to trigger the expression of inducible nitric oxide (iNOS). We propose IL-17 and NO modulation could be relevant forms of therapy for autoimmune disorders” [260]
“Together, these data indicate that antioxidant modulation of nitric oxide might be a feasible therapeutic tool to interfere with development of autoimmune diabetes” [261]
 Clinical Strategies to Support the Nitric Oxide System
Clinical Strategies to Support the Nitric Oxide System
The following compounds have been shown in the literature to modulate nitric oxide isomers:
- Adenosine 5’-triphosphate
- Huperzine A
- Vinpocetine
- L-alpha-glycerylphosphosphorylcholine
- Alpha-ketogluteric acid
- Xanthinol niacinate
- L-acetylcarnitine
 ATP (Adenosine 5’-triphosphate)
ATP (Adenosine 5’-triphosphate)
ATP (Adenosine 5’-triphosphate) is the fuel source for all biochemical reactions. ATP can be found inside the cell (intracellular) or outside the cell (extracellular). Research has also clearly demonstrated that supplementation with ATP also increases extracellular and intracellular levels of ATP. [262-264] Extracellular ATP has been found to activate eNOS and nNOS. [265-268] More importantly, ATP does not activate iNOS activity and has even demonstrated suppressive iNOS activity. [269-271]
 Huperzine A
Huperzine A
Huperzine A is powerful compound for ideal nitric oxide isomer expression because it enhances acetylcholine activity necessary for eNOS and nNOS and it has powerful iNOS inhibiting properties. [272-273] Huperzine is a potent natural acetylcholine esterase inhibitor, leading to increased acetylcholine activity that is necessary for ideal eNOS and nNOS activity. [274] Not only does Huperzine A inhibit iNOS activity directly, but it also suppresses the same cytokines released during exercise that stimulate iNOS. [275-278]
 Vinpocetine
Vinpocetine
Vinpocetine is classified as a selective cyclic GMP phosphodiesterase (PDE) balancer and modulates vascular tone and Nitric Oxide release in system circulation. [279-282] The net effect of PDE inhibitors, such as vinpocetine, is to increase eNOS activity, leading to healthy vasodilation and blood delivery to peripheral tissues, such as muscles and brain, important for peak athletic performance and recovery. In addition to vinpocetine’s properties of eNOS activation, the compound has powerful influences on the neurotransmitter acetylcholine. Acetylcholine activity is critical for ideal eNOS receptor site activity. [283-285]
 Alpha-glycerylphosphorylcholine (Alpha GPC)
Alpha-glycerylphosphorylcholine (Alpha GPC)
Alpha-GPC is a phospholipid metabolite that is isolated from lecithin. It is absorbed extremely well by the gastrointestinal tract and is used for the synthesis of acetylcholine. Oral intake of Alpha GPC has been shown to increase acetylcholine levels in the body. [286-287] Acetylcholine activity promotes nitric oxide synthase activity, and nitric oxide promotes acetylcholine activity that is responsible for eNOS vascular dilation and nNOS synaptic activity. [288-293]
 Alpha-Ketoglutaric Acid (AKG)
Alpha-Ketoglutaric Acid (AKG)
Alpha-ketoglutaric acid (AKG) is a citric cycle intermediate and can be used to help produce nitric oxide via the urea cycle and metabolize harmful nitrogen out of the body. It is also an intermediate precursor for metabolites that are used by the mitochondria to produce energy. AKG supplementation has been shown to have positive modulating activity on nitric oxide and iNOS. [294] AKG supplementation has been shown to help modulate nitrogen balance and preserve protein synthesis after cell injury. [295-297]
 Xanthinol Niacinate
Xanthinol Niacinate
Xanthinol niacinate has very powerful vascular-enhancing properties and the ability to improve peripheral hemodynamics. [298] It has demonstrated properties to increase eNOS by its nicotinate component activity on the acetylcholine receptors. [299] A human subject study comparing the effects of a placebo and xanthinol niacinate on exercise tolerance found that xainthinol niacinate significantly increased exercise tolerance at 1 hour and 1-9 hours respectively from ingesting the supplement. [300] Clinical research has found that xanthinol niacinate has antioxidant properties that may lead to decreased iNOS activities. Several research studies have found it improves microcirculation and aids in healing. [301-304]
 Acetyl L-Carnitine (ALCar)
Acetyl L-Carnitine (ALCar)
Acetyl L-carnitine is composed of acetic acid and L-carnitine bound together and has very close structural similarity to acetylcholine. Research has shown that ALCAR mimics acetylcholine activity and has shown effectiveness in acetycholine mediated pathways. [305-308] Acetylcholine activity promotes nitric oxidesynthase activity, and nitric oxide promotes acetycholine activity that is responsible for eNOS vascular dilation and nNOS synaptic activity. [309-314]
Lifestyle Strategies for Autoimmunity
If individuals have autoimmunity or are at risk of autoimmunity, they must focus on the proper lifestyle and nutritional strategies to decrease the expression of this condition. Patients with autoimmunity must know they will have bad days. Bad days may be triggered by immune activation from various scenarios, such as stress, lack of sleep, blood glucose fluctuations, food sensitivities, etc. It is important for them to realize what mechanisms can promote their autoimmunity and what mechanisms can dampen their autoimmunity so that they can be empowered to improve their quality of life and slow down self-tissue destruction and the progression of the disease.
 Lifestyle Factors that Promote TH-17 Responses and Autoimmune Reactions
Lifestyle Factors that Promote TH-17 Responses and Autoimmune Reactions
In addition to personal internal stressors, external stressors—such as their job, strict deadlines, unrealistic goals or any environment that places great demands on them—activates their TH-17 pathway, which promotes autoimmunity. The physiologic stress response that occurs from mental or physical stress increases pro-inflammatory cytokines such as IL-2, IL-4, IL-6 and IL-10 which activates the HPA axis and increases production of epinephrine and cortisol and activates the TH-17 pathways that then promote autoimmunity. [315-323] People with autoimmunity must take themselves out of a stressful environment even if it will effect their finances, relationships, commitments, etc. or they are at increased risk of progression of their autoimmune disease. Stress modification is a significant factor of autoimmunity expression, but completely overlooked by both alternative and conventional healthcare professionals today. They must create an environment that is protective and supportive for them so they can focus on their passions and increase opioid activity. This is necessary to break the vicious cycles of ill health they have established and help return them to better health
 Lifestyle Strategies that Support TH-3 Cells and Dampen Autoimmune Reactions
Lifestyle Strategies that Support TH-3 Cells and Dampen Autoimmune Reactions
Recall that regulatory T-cells (TH-3 cells) are very important for regulation of proper immune responses. The release of natural opioids by the body activates TH-3 cells and promotes anti-inflammatory responses. [324-328] TH-3 cells are loaded with opioid receptors and as the body releases them, they activate the TH-3 cells that shift autoimmunity into suppression. The best example of opioid release is the feeling people get after they exercise. In order to get the best exercise, the exercise has to be challenging. Just walking on the treadmill passively is not going to get the best opioid response. They must make the exercise challenging enough to get their heart rate up and push themselves when they want to quit—this will create the opioid release. The key is to push themselves hard enough to get the best “exercise high” they can get after their workout, without creating an injury or overtraining. [329-333]
 Being able to recover quickly after exercise is also very important and sets up the body chemistry to activate TH-3 without activating TH-17, which is critical for a healthy immune response. In addition to creating an “exercise high”, they want to create feelings that make them “high on life”. A positive mental attitude, love, appreciation for life, positive mental reflection, all increase opioids that make a person “high on life”. On the other hand, a negative attitude, violence, poor relationships, internal mental stress, all promote increased production of –pro-inflammatory cytokines which activate the TH-17 pathway and promote autoimmunity.
Being able to recover quickly after exercise is also very important and sets up the body chemistry to activate TH-3 without activating TH-17, which is critical for a healthy immune response. In addition to creating an “exercise high”, they want to create feelings that make them “high on life”. A positive mental attitude, love, appreciation for life, positive mental reflection, all increase opioids that make a person “high on life”. On the other hand, a negative attitude, violence, poor relationships, internal mental stress, all promote increased production of –pro-inflammatory cytokines which activate the TH-17 pathway and promote autoimmunity.
Summary of Lifestyle Modulation of Autoimmunity
Adverse Mechanisms which Promote Autoimmunity
- Excess stress
- Physical overtraining
- Lack of sleep
- Drops in blood glucose
- Poor social relationships
 Beneficial Mechanisms which Dampen Autoimmunity
Beneficial Mechanisms which Dampen Autoimmunity
- Love/appreciation
- Positive attitude
- Adequate exercise
- Adequate sleep
- Stable blood glucose
- Social/physical interaction
Dietary Strategies for Autoimmunity
Here are three common pathophysiologic stress responses that promote autoimmunity:
- Blood sugar fluctuations
- Gluten and cross-reactive foods
- Intestinal permeability
 In addition to internal and external stressors, people with autoimmunity must decrease their physiologic stress response by optimizing their diet and eating patterns. Maintaining stable glucose levels and avoiding blood sugar spikes is important to minimize inflammatory responses. Autoimmune individuals must also avoid eating gluten or any foods that cross-react with gluten (discussed below). They must also try to improve their leaky gut/intestinal permeability condition by sticking with a low-carb/paleo diet and eating frequently throughout the day. Making the lifestyle and dietary changes as discussed below can lead to significant changes in their overall health and function. These changes will also dampen immune responses and potentially slow down the progression of the autoimmunity. Let’s discuss each of these in a bit more detail.
In addition to internal and external stressors, people with autoimmunity must decrease their physiologic stress response by optimizing their diet and eating patterns. Maintaining stable glucose levels and avoiding blood sugar spikes is important to minimize inflammatory responses. Autoimmune individuals must also avoid eating gluten or any foods that cross-react with gluten (discussed below). They must also try to improve their leaky gut/intestinal permeability condition by sticking with a low-carb/paleo diet and eating frequently throughout the day. Making the lifestyle and dietary changes as discussed below can lead to significant changes in their overall health and function. These changes will also dampen immune responses and potentially slow down the progression of the autoimmunity. Let’s discuss each of these in a bit more detail.
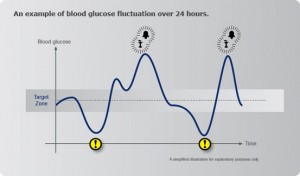 Blood Sugar Fluctuations
Blood Sugar Fluctuations
The most common and significant biochemical stressor today is dysglycemia or poor glucose control from diet and lifestyle. Individuals with autoimmunity need to make sure their blood glucose levels are not going up and down all day like a roller coaster. Numerous studies have shown the effects of oxidative stress and upregulation of pro-inflammatory cytokines when glucose levels fluctuate. [334-338] Studies also show there is a decrease in oxidative stress and inflammation by blunting glucose fluctuations. [339]
When blood glucose is low from missing meals or eating meals that are not sufficient in fiber or protein, pro-inflammatory cytokine production (ie: IL-6) increases to raise blood glucose. [340-345] IL-6 not only activates the endocrine system (to produce more adrenal hormones to raise blood glucose) but recall it also stimulates the TH-17 pathways that activate autoimmunity, which we discussed above. [346]
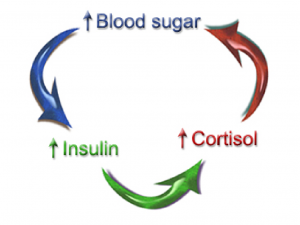 Insulin Resistance
Insulin Resistance
If they get tired after meals, they are likely eating too much food for their body to handle, particularly excess carbohydrates with insufficient protein and/or are not getting enough exercise. This type of person is often overweight and will often show elevations of fasting blood glucose and triglycerides in their labwork. These are all signs of insulin resistance, as discussed in part 1 of this series. This is an issue of the body’s cells not responding to insulin the way they should. Recall that insulin is the hormone that allows the cells to uptake glucose and lower glucose levels in the blood.
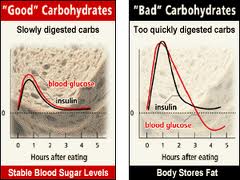 Reactive Hypoglycemia
Reactive Hypoglycemia
If someone gets shaky, lightheaded or irritable when they go too long without eating, this is a sign of their blood glucose levels falling too low. Symptoms are often relieved after eating. If they feel better after they eat, they are either going too long without eating or are not getting enough fiber and protein to stabilize their blood sugar. These people will often show low to normal fasting blood glucose levels and tend to be thinner and often have low blood pressure. Either way, symptoms that improve or worsen after eating meals is not a good sign and indicates some type of blood glucose regulation issue that needs to be addressed to improve autoimmunity.
Signs of Blood Glucose Issues and Dietary Needs
- Fatigue between meals
- Eating relieves fatigue
- Crash in the afternoon
- Difficulty staying asleep
- Shaky, lightheaded or irritable between meals
Dietary Recommendations
- High-protein meals
- Avoid simple sugars
- Eat every 2-3 hours
- Fatigue after meals
- Sugar cravings after meals
- Need for coffee or stimulants after meals
Dietary need
- Avoid or limit carbohydrates
- Eat small meal portions
 Gluten and Cross-Reactive Foods
Gluten and Cross-Reactive Foods
Avoiding food allergens and inflammatory foods is critical to minimizing the triggering of immune responses and tissue destruction in autoimmunity. By far, the most inflammatory food being consumed in the U.S. is gluten. Non-celiac gluten sensitivity has been linked with several types of disease, including autoimmune disease, as we discussed in part 4 of this series [347-353] but it is important to understand that gluten can make any autoimmune disease worse in individuals who are gluten-sensitive because of the inflammatory nature of gluten. [354-358]
As we saw in part 4 of this series, gluten sensitivity is extremely common, potentially affecting a much larger percentage of the population than we realize. We do not know exactly how common gluten reactions actually are (because conventional blood testing for this is not reliable since it is only testing for one fraction of wheat seen in celiac disease, as discussed in part 4) but we do know that approximately 30-40% of the normal population have the HLA-DQ2 or DQ8 genotype (the genotype associated with celiac disease) [359] and one estimate is that gluten sensitivity affects 29% of the normal population. [360]
We also know that gluten sensitivity presents as a wide variety of symptoms [361], and has been implicated in the development of intestinal permeability or leaky gut syndrome [362]. As we learned in part 5, intestinal permeability is now being theorized to be one of the primary mechanisms in the pathogenesis (development of disease) of autoimmune disease. [363-366] So there is a clear connection between gluten sensitivity, leaky gut and the development and progression of autoimmune disease.
 “Why am I Not Feeling Better on a Gluten-Free Diet?”
“Why am I Not Feeling Better on a Gluten-Free Diet?”
Once a patient is diagnosed as Celiac Disease (CD) or showing signs (or suspected of having) gluten sensitivity, he/she is instructed to adhere to a gluten-free diet (GFD). There are many resources that can help the patient with this seemingly difficult process. However, a significant percentage of these patients will continue to have gluten-like complaints even after being on a GFD for months. [367] Why is that?
“Gluten-Free” Products are Not Necessarily Gluten-Free
Most countries define “gluten-free” products based on the recommendation of the Food and Agricultural Organization of the United Nations and World Health Organization. This codex allows the inclusion of up to 0.3% protein from gluten containing grains in foods labeled “gluten-free.” In some gluten-sensitive individuals who are exposed to 0.3% gluten protein, the immune system can still recognize and react to the protein. [368-369]
 Hidden Sources of Gluten
Hidden Sources of Gluten
Wheat/gliadin is commonly added to foods as a “hidden” ingredient in various types of food additives such as soy sauce, food starches, food emulsifiers, malt extract and dextrins. These are often referred to as “natural flavors”, “food starches” or “food stabilizers.” A complete understanding of ‘hidden wheat/gluten’ sources is necessary to have a better outcome with the GFD. Additionally, education of gluten-containing grains, such as rye, barley, Polish wheat and spelt, is also important.
Hidden sources of gluten:
- Soy sauce
- Salad dressings
- Food starches
- Food emulsifiers
- Food stabilizers
- Artificial food coloring
- Malt extract, flavor, syrup
- Dextrins
 Cross-Reactivity with Gluten
Cross-Reactivity with Gluten
Another major reason for gluten-related symptoms while on a GFD is because there exists antigenic similarity, or cross-reaction, among many grains, and other dietary proteins with gluten. In order to understand this concept of cross-reactivity, let’s review some basic concepts of immunology.
Immune responses to food occur when antibodies of the immune system recognize and bind to antigens (specific to that food) made of proteins which are composed of a sequence of amino acids. There are a number of foods that have been identified to have a similar amino acid sequence as gluten, including dairy. In fact, resarch indicates about 50% of patients with celiac disease have problems digesting dairy and may, therefore, contribute to persistent symptoms in patients who are on a GFD. [370] Gluten antibodies can bind to these non-gluten food antigens initiating inflammatory responses similar to those seen with gluten. This type of response is termed “cross-reactivity” and has been clearly-established in the literature. [371-378] 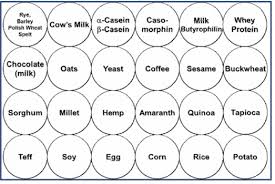 This is a big problem for people who are gluten-sensitive because their immune systems “cross-react” to many types of food as if they were consuming gluten and cause major problems for these people, especially if they already have autoimmunity.
This is a big problem for people who are gluten-sensitive because their immune systems “cross-react” to many types of food as if they were consuming gluten and cause major problems for these people, especially if they already have autoimmunity.
Research has indicated that identifying these foods appears to be a valuable adjunct in the diagnosis and follow-up of diagnosed celiac disease, both in children and adults. Monitoring these antibodies may be particularly helpful in evaluating the response of patients on a gluten- and cross-reactive food-free diet. [379] The following is a list of foods which have been shown to cross-react with gluten.
Gluten Cross-Reactive Foods
|
 Development of Additional Food Sensitivities
Development of Additional Food Sensitivities
Additionally, patients who are new to the GFD encounter new foods and/or over-consume old favorites to compensate for the lack of wheat in the diet. Gluten-free cookies, crackers, breads and cakes often contain copious amounts of rice, amaranth, sorghum and other substitutes. Some of these new-to-the-patient foods may illicit an adverse reaction. Other foods that are often introduced to the patient on the GFD are quinoa, buckwheat and hemp. Some patients may turn to the “ancient” grains (Polish wheat, spelt, barley, rye), not knowing that these contain gluten. Another problem patients often face on the GFD is the over-consumption of another starch to make up for the loss of wheat. They turn to potato, rice or corn as a substitute. This can lead to the development of a new sensitivity or the enhancement of old sensitivities. [380-386]
 Laboratory Testing for Gluten Sensitivity and Gluten Cross-Reactive Foods
Laboratory Testing for Gluten Sensitivity and Gluten Cross-Reactive Foods
In part 4 of this series, we discussed the inappropriateness and unreliability of conventional lab testing (anti-gliadin and anti-tissue transglutaminase 2) for gluten sensitivity. This test was designed to detect celiac disease, not all the other types of responses now known to occur in gluten sensitivity. As a result, this test only tests for a limited number of antibody responses and we learned that there are many other types of reactions to gluten as well as other wheat fractions that are not detected in this test that can result in various illnesses. [387] Fortunately, there is a reliable comprehensive gluten sensitivity test available through Cyrex Labs which tests for IgA and IgG antibodies to twelve different reactions to wheat: Array 3x: Wheat/Gluten Proteome Reactivity and Autoimmunity test.
 In addition to performing reliable testing for gluten sensitivity in individuals where gluten sensitivity is suspected, it is advised to check for immune responses to gluten cross-reactive foods in any individual shown to have gluten sensitivity when symptoms persist after initiation of a gluten-free diet (GFD). Cyrex Labs offers a comprehensive test which tests for immune responses to 24 different cross-reactive foods or foods that patients often develop sensitivity to after initiating a GFD, as discussed above: Array 4: Gluten-Associated Cross-Reactive Foods and Foods Sensitivity test. In my practice, I recommend this test for anyone who is known to have gluten sensitivity but continues to have gluten-related symptoms, even after initiating a GFD.
In addition to performing reliable testing for gluten sensitivity in individuals where gluten sensitivity is suspected, it is advised to check for immune responses to gluten cross-reactive foods in any individual shown to have gluten sensitivity when symptoms persist after initiation of a gluten-free diet (GFD). Cyrex Labs offers a comprehensive test which tests for immune responses to 24 different cross-reactive foods or foods that patients often develop sensitivity to after initiating a GFD, as discussed above: Array 4: Gluten-Associated Cross-Reactive Foods and Foods Sensitivity test. In my practice, I recommend this test for anyone who is known to have gluten sensitivity but continues to have gluten-related symptoms, even after initiating a GFD.
Intestinal Permeability Dietary Restrictions
Finally, as discussed above, it is important to avoid foods that tend to aggravate or worsen intestinal permeability when trying to repair the gut barrier and decrease autoimmune responses. Sugars and high glycemic foods can create more damage to the intestinal lining and increase permeability. Gluten (and other grains that cross-react with gluten), dairy and soy can also cause additional inflammation and lead to further damage to the epithelial lining of the GI tract. Lectins (found in grains, legumes, nightshade plants and dairy) have also been shown to damage this epithelial ling. It is important to implement an aggressive and complete dietary program to break the vicious cycle of leaky gut and minimize autoimmune reactions. Here is a list of foods shown to aggravate or worsen intestinal permeability as well as the foods that are generally safely tolerated in individuals with this condition.
 Foods to Avoid
Foods to Avoid
- Sugars: including corn syrup, high fructose corn syrup, molasses, honey, chocolate candy, etc.
- High glycemic fruits: watermelon, mango, pineapple, raisins and canned fruit
- Grains: including gluten, wheat, oats, rice, barley, buckwheat, soy, corn, wheat germ, spelt, amaranth, kamut, millet, and quinoa, etc.
- Gluten-containing compounds: including processed salad dressings, ketchup, soy sauce, barbecue sauce, condiments, modified starches, etc.
 Dairy: including milk, whey, eggs, cheeses, creams, and mayonnaise, etc.
Dairy: including milk, whey, eggs, cheeses, creams, and mayonnaise, etc.- Soy: including soy milk, soy sauce, soy protein, etc.
- Alcohol: including beer, wine, sake, cognac, liquors, etc.
- Lectins: including nuts, beans, soy, potatoes, tomato, eggplant, pepper, peanut oil, soy oil, etc.
- Coffee
- Processed foods
- Canned foods
 Foods to Eat
Foods to Eat
- Most vegetables (except tomato, potatoes and mushrooms): including asparagus, spinach, lettuce, broccoli, beets, cauliflower, carrots, celery artichokes, garlic, onions, zucchini, squash, rhubarb, cucumbers, turnips, watercress, etc.
- Fermented foods: including sauerkraut, kimchi, pickled ginger, mixed pickle, coconut yogurt, kombucha tea, etc.
 Meats: including fish, chicken, beef, lamb, organ meats, etc.
Meats: including fish, chicken, beef, lamb, organ meats, etc.- Low glycemic foods: apricot, plum apple, peach, pear, cherries, berries, etc.
- Coconut: including coconut oil, coconut bitter, coconut milk, etc.
- Herbal teas
- Olives and olive oil
Cyrex Labs Array 2: Intestinal Permeability Antigenic Screen
 Cyrex Labs offers a reliable intestinal barrier antigenic test which is currently the only commercial lab available that measures the immunological response against the proteins that compose the intestinal barrier and accepted markers of permeability, such as lipopolysaccharides (LPS). The profile includes actin/myosin network IgG, occludin/zonulin IgG, IgA, IgM and lipopolysaccharides (LPS) IgG, IgA, and IgM. Measurements of these antibodies can be used as reliable markers for intestinal permeability. This condition is extremely important to assess in individuals who have autoimmunity or autoimmunity is suspected, or patients that present with common signs or symptoms associated with intestinal permeability as described above and whom the development of autoimmunity is a concern.
Cyrex Labs offers a reliable intestinal barrier antigenic test which is currently the only commercial lab available that measures the immunological response against the proteins that compose the intestinal barrier and accepted markers of permeability, such as lipopolysaccharides (LPS). The profile includes actin/myosin network IgG, occludin/zonulin IgG, IgA, IgM and lipopolysaccharides (LPS) IgG, IgA, and IgM. Measurements of these antibodies can be used as reliable markers for intestinal permeability. This condition is extremely important to assess in individuals who have autoimmunity or autoimmunity is suspected, or patients that present with common signs or symptoms associated with intestinal permeability as described above and whom the development of autoimmunity is a concern.
References
- Nuclear factor-kB—a pivotal transcription factor in chronic inflammatory diseases. NF-kB Inflammatory Amplifying Loop. N Eng J Med. 1997;336:1066-1071.
- Nuclear factor-kB—a pivotal transcription factor in chronic inflammatory diseases. NF-kB Inflammatory Amplifying Loop. N Eng J Med. 1997;336:1066-1071.
- Return to homeostasis: downregulation of NF-kB responses. Nat Immunol. 2011 Jun 19;12(8):709-714
- NF-kB in type 1 diabetes. Inflamm Allergy Drug Targets. 2011 Jun;10(3):208-217.
- NF-kB as a therapeutic target in autoimmune disease. Expert Opin Ther Targets. 2007 Feb;11(2):111-122
- Naturally occurring NF-kB inhibitors. Mini Rev Med Chem. 2006 Aug;6(8):945-951.
- Curcumin has bright prospects for the treatment of multiple sclerosis. Int Immunopharmacol. 2011 Mar;11(3):323-330
- Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. Bengmark S. JPEN J Parenter Enteral Nutr. 2006 Jan-Feb;30(1):45-51. Review.
- Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Baliga MS, Joseph N, Venkataranganna MV, Saxena A, Ponemone V, Fayad R. Food Funct. 2012 Nov;3(11):1109-17. doi: 10.1039/c2fo30097d. Review.
- Curcumin ameliorates cardiac inflammation in rats with autoimmune myocarditis. Biol Pharm Bull. 2011;34(7):974-979 JPEN J Parenter Enteral Nutr. 2006 Jan-Feb;30(1):45-51. Review.
- “Spicing up” of the immune system by curcumin. Jagetia GC, Aggarwal BB. J Clin Immunol. 2007 Jan;27(1):19-35. Epub 2007 Jan 9. Review.
- The targets of curcumin. Curr Drug Targets. 2011 Mar 1;12(3):332-347.
- Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J neuroopthalmol. 2010 Dec;30(4):328-339
- NF-kB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010 Apr 2;394(2):360-365
- Resveratrol suppresses IL-1β-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential use for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol. 2008 Dec 1;76(11):1426-39
- Resveratrol ameliorates experimental autoimmune myocarditis. Circ J. 2007 Mar;71(3):397-404
- Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutrition and Cancer. 2009;61(6):847–854.
- Li T, Fan G-X, Wei W, Li T, Yuan Y-K. Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo. International Immunopharmacology. 2007;7:1221–1231.
- Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutrition and Metabolism. 2008;5(1):17–30.
- Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 1977;2:432-434.
- Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Curr Med Chem. 2006:13(15): 1749-55
- Nazzareno Ballatori, Suzanne M. Krance, Sylvia Notenboom, Shujie Shi, Kim Tieu, Christine L. Hammond. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009 March; 390(3): 191–214.
- Correlation of lipid peroxidation and glutathione levels with severity of systemic lupus erythmatosus: a pilot study from single center. J Pharm Pharm Sci. 2008;11(3):30-4
- Nazzareno Ballatori, Suzanne M. Krance, Sylvia Notenboom, Shujie Shi, Kim Tieu, Christine L. Hammond. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009 March; 390(3): 191–214.
- Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev 2005;4:288-314.
- Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing TH2 and TH17 cell development. Antioxid Redox Signal. 2010 Jun 6.
- Odom RY, Dansby MY, Rollins-Hairston AM, Jackson KM, Kirlin WG. Phytochemical induction of cell cycle arrest by glutathione oxidation and reversal by N-acetylcysteine in human colon carcinoma cells. Nutr Cancer. 2009;61(3):332-9.
- Soghier LM, Brion LP. Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD004869.
- Badaloo A, Reid M, Forrester T, Heird WC, Jahoor F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am J Clin Nutr. 2002 Sep;76(3):646-52.
30. Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Machado Cesar LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant 2004;19:1803-1807.
31. Shimada K, Murayama T, Yokode M, Kita T, Uzui H, Ueda T, Lee JD, Kishimoto C. N-acetylcysteine reduces the severity of atherosclerosis in apolipoprotein E-deficient mice by reducing superoxide production. Circ J 2009;73:1337-1341.
32. Moldeus P, Cotgreave IA, Berggren M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration 1986;50 Suppl 1:31-42.
33. Prescott LF, Park J, Ballantyne A, Adriaenssens P, Proudfoot AT. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet 1977;2:432-434.
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as adietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009 Oct;1790(10):1149-60. Epub 2009 Aug 4.
35. Kinnunen S, Oksala N, Hyyppä S, Sen CK, Radak Z, Laaksonen DE, Szabó B, Jakus J, Atalay M. Alpha-Lipoic acid modulates thiol antioxidant defenses and attenuates exercise-induced oxidative stress in standardbred trotters. Free Radic Res. 2009 Aug;43(8):697-705.
36. Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, Carlson DA, Münch G. Lipoic acid as an anti-infl ammatory and neuroprotective treatment for Alzheimer’s disease. Adv Drug Deliv Rev. 2008 Oct-Nov;60(13-14):1463-70.
37. Suh JH, Moreau R, Heath SH, Hagen TM. Dietary supplementation with (R)-alpha-lipoic acid reverses the age-related accumulation of iron and depletion of antioxidants in the rat cerebral cortex. Redox Rep. 2005;10(1):52-60.
38. Hill AS, Rogers QR, O’Neill SL, Christopher MM. Effects of dietary antioxidant supplementation before and after oral acetaminophen challenge in cats. Am J Vet Res. 2005 Feb;66(2):196-204.
39. Zicker SC, Hagen TM, Joisher N, Golder C, Joshi DK, Miller EP. Safety of long-term feeding of dl-alpha-lipoic acid and its effect on reduced glutathione:oxidized glutathione ratios in beagles. Vet Ther. 2002 Summer;3(2):167-76.
40. Jones W, Li X, Qu ZC, Perriott L, Whitesell RR, May JM. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic Biol Med. 2002 Jul 1;33(1):83-93.
41. Miquel J. Can antioxidant diet supplementation protect against agerelated
mitochondrial damage? Ann N Y Acad Sci. 2002 Apr;959:508-16.
42. Khanna S, Atalay M, Laaksonen DE, Gul M, Roy S, Sen CK. Alpha-lipoic acid supplementation: tissue glutathione homeostasis at rest and after exercise. J Appl Physiol. 1999 Apr;86(4):1191-6.
43. Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H, Tritschler HJ, Flohé L, Packer L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6(3):321-38.
44. Kul M, Vurucu S, Demirkaya E, Tunc T, Aydinoz S, Meral C, Kesik V, Alpay F. Enteral glutamine and/or arginine supplementation have favorable effects on oxidative stress parameters in neonatal rat intestine. J Pediatr Gastroenterol Nutr. 2009 Jul;49(1):85-9.
45. Fan YP, Yu JC, Kang WM, Zhang Q. Effects of glutamine supplementation on patients undergoing abdominal surgery. Chin Med Sci J. 2009 Mar;24(1):55-
46. Todorova V, Vanderpool D, Blossom S, Nwokedi E, Hennings L, Mrak R, Klimberg VS. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition. 2009 Jul-Aug;25(7-8):812-7.
47. Cruzat VF, Tirapegui J. Effects of oral supplementation with glutamine and alanyl-glutamine on glutamine, glutamate, and glutathione status in trained rats and subjected to long-duration exercise. Nutrition. 2009 Apr;25(4):428-35.
48. Kaufmann Y, Todorova VK, Luo S, Klimberg VS. Glutamine affects glutathione recycling enzymes in a DMBA-induced breast cancer model. Nutr Cancer. 2008;60(4):518-25.
49. Xue H, Sawyer MB, Field CJ, Dieleman LA, Murray D, Baracos VE. Bolus oral glutamine protects rats against CPT-11-induced diarrhea and differentially activates cytoprotective mechanisms in host intestine but not tumor. J Nutr. 2008 Apr;138(4):740-6.
50. Mok E, Constantin B, Favreau F, Neveux N, Magaud C, Delwail A, Hankard R. L-glutamine administration reduces oxidized glutathione and MAP kinase signaling in dystrophic muscle of mdx mice. Pediatr Res. 2008 Mar;63(3):268-73.
51. Kaufmann Y, Klimberg VS. Effect of glutamine on gut glutathione fractional release in the implanted tumor model. Nutr Cancer. 2007;59(2):199-206.
52. Belmonte L, Coëffi er M, Le Pessot F, Miralles-Barrachina O, Hiron M, Leplingard A, Lemeland JF, Hecketsweiler B, Daveau M, Ducrotté P, Déchelotte P. Effects of glutamine supplementation on gut barrier, glutathione content and acute phase response in malnourished rats during infl ammatory shock. World J Gastroenterol. 2007 May 28;13(20):2833-40.
53. Jackson AA, Gibson NR, Lu Y, Jahoor F. Synthesis of erythrocyte glutathione in healthy adults consuming the safe amount of dietary protein.Am J Clin Nutr.2004 Jul;80(1):101-7.
54. Melis GC, ter Wengel N, Boelens PG, van Leeuwen PA. Glutamine: recent developments in research on the clinical significance of glutamine. Curr Opin Clin Nutr Metab Care. 2004 Jan;7(1):59-70.
55. Johnson AT, Kaufmann YC, Luo S, Todorova V, Klimberg VS. Effect of glutamine on glutathione, IGF-I, and TGF-beta 1. J Surg Res. 2003 May 15;111(2):222-8.
56. Manhart N, Vierlinger K, Spittler A, Bergmeister H, Sautner T, Roth E. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer’s patches in endotoxemic mice. Ann Surg. 2001 Jul;234(1):92-7.
57. Le Bricon T. Effect of glutamine supplementation on protein metabolism
and glutathione in tumor-bearing rats. Clin Nutr. 1996 Aug;15(4):211.
58. Wichtel JJ, Thompson KG, Williamson NB. Serum glutathione peroxidase activity reflects short-term increases in selenium intake in goats. N Z Vet J. 1996 Aug;44(4):148-50
59. Delmas-Beauvieux MC, Peuchant E, Couchouron A, Constans J, Sergeant C, Simonoff M, Pellegrin JL, Leng B, Conri C, Clerc M. The enzymatic antioxidant system in blood and glutathione status in human immunodeficiency virus (HIV)-infected patients: effects of supplementation with selenium or beta-carotene. Am J Clin Nutr. 1996 Jul;64(1):101-7. Erratum in: Am J Clin Nutr 1996 Dec;64(6):971.
60. Winnefeld K, Schirrmeister W, Thiele R, Sperschneider H, Klinger G. Selenium and antioxidant status in various diseases. Med Klin (Munich). 1995 Jan 15;90 Suppl 1:7-9
61. Bellisola G, Perona G, Galassini S, Moschini G, Guidi GC. Plasma selenium and glutathione peroxidase activities in individuals living in the Veneto region of Italy. J Trace Elem Electrolytes Health Dis. 1993 Dec;7(4):242-4.
62. Bedwal RS, Nair N, Sharma MP, Mathur RS. Selenium–its biological perspectives. Med Hypotheses. 1993 Aug;41(2):150-9. PubMed
63. Zachara BA, Mikolajczak J, Trafi kowska U. Effect of various dietary selenium (Se) intakes on tissue Se levels and glutathione peroxidase activities in lambs. Zentralbl Veterinarmed A. 1993 May;40(4):310-8.
64. Wilke BC, Vidailhet M, Favier A, Guillemin C, Ducros V, Arnaud J, Richard MJ. Selenium, glutathione peroxidase (GSH-Px) and lipid peroxidation products before and after selenium supplementation. Clin Chim Acta. 1992 Apr 30;207(1-2):137-42.
65. Bellisola G, Galassini S, Moschini G, Poli G, Perona G, Guidi G. Selenium and glutathione peroxidase variations induced by polyunsaturated fatty acids oral supplementation in humans. Clin Chim Acta. 1992 Jan 31;205(1-2):75-85.
66. Pearson DJ, Day JP, Suarez-Mendez VJ, Miller PF, Owen S, Woodcock A. Human selenium status and glutathione peroxidase activity in northwest England. Eur J Clin Nutr. 1990 Apr;44(4):277-83.
67. Michaëlsson G, Edqvist LE. Erythrocyte glutathione peroxidase activity in acne vulgaris and the effect of selenium and vitamin E treatment. Acta Derm Venereol. 1984;64(1):9-14. PubMed PMID: 6203294.
68. Doni MG, Avventi GL, Bonadiman L, Bonaccorso G. Glutathione peroxidase, selenium, and prostaglandin synthesis in platelets. Am J Physiol. 1981 May;240(5):H800-3.
69. Hafeman DG, Sunde RA, Hoekstra WG. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974 May;104(5):580-7.
70. Ko WS, Hsu SL, Chyau CC, Chen KC, Peng RY. Compound Cordyceps TCM-700C exhibits potent hepatoprotective capability in animal model. Fitoterapia. 2010 Jan;81(1):1-7.
71. Ji DB, Ye J, Li CL, Wang YH, Zhao J, Cai SQ. Antiaging effect of Cordyceps sinensis extract. Phytother Res. 2009 Jan;23(1):116-22.
72. Wang YH, Ye J, Li CL, Cai SQ, Ishizaki M, Katada M. [An experimental study on anti-aging action of Cordyceps extract]. Zhongguo Zhong Yao Za Zhi. 2004 Aug;29(8):773-6.
73. Liu Y, Wu C, Li C. [Anti-oxidation of Paecilomyces Sinensis (S. Pnov.)]. Zhongguo Zhong Yao Za Zhi. 1991 Apr;16(4):240-2, 256.
74. Haleagrahara N, Ponnusamy K. Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced parkinsonism in aged Sprague-Dawley rats. J Toxicol Sci.2010;35(1):41-7.
75. Shinomol GK, Ravikumar H; Muralidhara. Prophylaxis with Centella asiatica confers protection to prepubertal mice against 3-nitropropionicacid-induced oxidative stress in brain. Phytother Res. 2009 Nov 26.
76. Shinomol GK, Muralidhara. Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology. 2008 Nov;29(6):948-57.
77. Shinomol GK, Muralidhara. Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and its in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine. 2008 Jun 5.
78. Flora SJ, Gupta R. Benefi cial effects of Centella asiatica aqueous extract against arsenic-induced oxidative stress and essential metal status in rats. Phytother Res. 2007 Oct;21(10):980-8.
79. Ramanathan M, Sivakumar S, Anandvijayakumar PR, Saravanababu C, Pandian PR. Neuroprotective evaluation of standardized extract of Centella asciatica in monosodium glutamate treated rats. Indian J Exp Biol. 2007 May;45(5):425-31.
80. Gupta R, Flora SJ. Effect of Centella asiatica on arsenic-induced oxidative stress and metal distribution in rats. J Appl Toxicol. 2006 May-Jun;26(3):213-22.
81. Jayashree G, Kurup Muraleedhara G, Sudarslal S, Jacob VB. Antioxidant activity of Centella asiatica on lymphoma-bearing mice. Fitoterapia. 2003 Jul;74(5):431-4.
82. Gupta YK, Veerendra Kumar MH, Srivastava AK. Effect of Centella asiatica on pentylenetetrazole-induced kindling, cognition and oxidative stress in rats. Pharmacol Biochem Behav. 2003 Feb;74(3):579-85.
83. Veerendra Kumar MH, Gupta YK. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J Ethnopharmacol. 2002 Feb;79(2):253-60.
84. Lee MK, Kim SR, Sung SH, Lim D, Kim H, Choi H, Park HK, Je S, Ki YC. Asiatic acid derivatives protect cultured cortical neurons from glutamate-induced excitotoxicity. Res Commun Mol Pathol Pharmacol. 2000 Jul-Aug;108(1-2):75-86.
85. Shaker E, Mahmoud H, Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010 Mar;48(3):803-6.
86. Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009 Sep;14(3):226-46.
87. Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, infl ammation and renal tumor promotionresponse. Invest New Drugs. 2009 Jul 10.
88. Lu P, Mamiya T, Lu LL, Mouri A, Zou L, Nagai T, Hiramatsu M, Ikejima T, Nabeshima T. Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice. Br J Pharmacol. 2009 Aug;157(7):1270-7.
89. Gharagozloo M, Moayedi B, Zakerinia M, Hamidi M, Karimi M, Maracy M, Amirghofran Z. Combined therapy of silymarin and desferrioxamine in patients with beta-thalassemia major: a randomized double-blind clinical trial. Fundam Clin Pharmacol. 2009 Jun;23(3):359-65.
90. Kim SH, Cheon HJ, Yun N, Oh ST, Shin E, Shim KS, Lee SM. Protective effect of a mixture of Aloe vera and Silybum marianum against carbon tetrachloride-induced acute hepatotoxicity and liver
fi brosis. J Pharmacol Sci. 2009 Jan;109(1):119-27.
91. Turgut F, Bayrak O, Catal F, Bayrak R, Atmaca AF, Koc A, Akbas A, Akcay A, Unal D. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int Urol Nephrol. 2008;40(2):453-60.
92. Ligeret H, Brault A, Vallerand D, Haddad Y, Haddad PS. Antioxidant and mitochondrial protective effects of silibinin in cold preservation-warm reperfusion liver injury. J Ethnopharmacol. 2008 Feb 12;115(3):507-14.
93. Toklu HZ, Tunali Akbay T, Velioglu-Ogunc A, Ercan F, Gedik N, Keyer-Uysal M, Sener G. Silymarin, the antioxidant component of Silybum marianum, prevents sepsis-induced acute lung and brain injury. J Surg Res. 2008 Apr;145(2):214-22.
94. Song Z, Song M, Lee DY, Liu Y, Deaciuc IV, McClain CJ. Silymarin prevents palmitate-induced lipotoxicity in HepG2 cells: involvement of maintenance of Akt kinase activation. Basic Clin Pharmacol Toxicol. 2007 Oct;101(4):262-8.
95. Toklu HZ, Tunali-Akbay T, Erkanli G, Yüksel M, Ercan F, Sener G. Silymarin, the antioxidant component of Silybum marianum, protects against burn-induced oxidative skin injury. Burns. 2007 Nov;33(7):908-16.
96. Kiruthiga PV, Shafreen RB, Pandian SK, Arun S, Govindu S, Devi KP. Protective effect of silymarin on erythrocyte haemolysate against benzo(a)pyrene and exogenous reactive oxygen species (H2O2) induced oxidative stress. Chemosphere. 2007 Jul;68(8):1511-8.
97. Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006 Nov;124(5):491-504.
98. Das SK, Vasudevan DM. Protective effects of silymarin, a milk thistle (Silybium marianum) derivative on ethanol-induced oxidative stress in liver. Indian J Biochem Biophys. 2006 Oct;43(5):306-11.
99. Edwards J, Grange LL, Wang M, Reyes E. Fetoprotectivity of the flavanolignan compound siliphos against ethanol-induced toxicity. Phytother Res. 2000 Nov;14(7):517-21.
100. Täger M, Dietzmann J, Thiel U, Hinrich Neumann K, Ansorge S. Restoration of the cellular thiol status of peritoneal macrophages from CAPD patients by the fl avonoids silibinin and silymarin. Free Radic Res. 2001 Feb;34(2):137-51
101. Valenzuela A, Aspillaga M, Vial S, Guerra R. Selectivity of silymarin on the increase of the glutathione content in different tissues of the rat. Planta Med. 1989 Oct;55(5):420-2.
102. Fahey JW, Talalay P. Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol 1999;37:973-979.
103. Barcelo S, Gardiner JM, Gescher A, Chipman JK. CYP2E1-mediated mechanism of anti-genotoxicity of the broccoli constituent sulforaphane. Carcinogenesis 1996;17:277-282.
104. Maheo K, Morel F, Langouet S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res 1997;57:3649-3652.
105. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175.
106. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms Ann N Y Acad Sci. 2009 May;1165:195-205
107. Noyer CM, Simon D, Borczuk A, Brandt LJ, Lee MJ, Nehra V. A double-blind placebo-controlled pilot study of glutamine therapy for abnormal intestinal permeability in patients with AIDS. Am J Gastroenterol. 1998 Jun;93(6):972-5.
108. Klimberg VS, Souba WW, Dolson DJ, Salloum RM, Hautamaki RD, Plumley DA, Mendenhall WM, Bova FJ, Khan SR, Hackett RL, et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990 Jul 1;66(1):62-8.
109. Chamorro S, de Blas C, Grant G, Badiola I, Menoyo D, Carabaño R. Effect of dietary supplementation with glutamine and a combination of glutamine-arginine on intestinal health in twenty-five-day-old weaned rabbits. J Anim Sci. 2010 Jan;88(1):170-80.
110. Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann N Y Acad Sci. 2009 May;1165:267-73
111. Kul M, Vurucu S, Demirkaya E, Tunc T, Aydinoz S, Meral C, Kesik V, Alpay F. Enteral glutamine and/or arginine supplementation have favorable effects on oxidative stress parameters in neonatal rat intestine. J Pediatr Gastroenterol Nutr. 2009 Jul;49(1):85-9.
112. Azuma H, Mishima S, Oda J, Homma H, Sasaki H, Hisamura M, Ohta S, Yukioka T. Enteral supplementation enriched with glutamine, fiber, and oligosaccharide prevents gut translocation in a bacterial overgrowth model. J Trauma. 2009 Jan;66(1):110-4.
113. Maes M, Leunis JC. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuro Endocrinol Lett. 2008 Dec;29(6):902-10.
114. Tian J, Hao L, Chandra P, Jones DP, Willams IR, Gewirtz AT, Ziegler TR. Dietary glutamine and oral antibiotics each improve indexes of gut barrier function in rat short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2009 Feb;296(2):G348-55.
115. Coëffier M, Claeyssens S, Lecleire S, Leblond J, Coquard A, Bôle-Feysot C, Lavoinne A, Ducrotté P, Déchelotte P. Combined enteral infusion of glutamine, carbohydrates, and antioxidants modulates gut protein metabolism in humans. Am J Clin Nutr. 2008 Nov;88(5):1284-90.
116. Wang WW, Qiao SY, Li DF. Amino acids and gut function. Amino Acids. 2009 May;37(1):105-10. Epub 2008 Aug 1. Review. PubMed PMID:18670730.
117. Xue H, Sawyer MB, Field CJ, Dieleman LA, Murray D, Baracos VE. Bolus oral glutamine protects rats against CPT-11-induced diarrhea and differentially activates cytoprotective mechanisms in host intestine but not tumor. J Nutr. 2008 Apr;138(4):740-6.
118. Vicario M, Amat C, Rivero M, Moretó M, Pelegrí C. Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J Nutr. 2007 Aug;137(8):1931-7.
119. Harsha WT, Kalandarova E, McNutt P, Irwin R, Noel J. Nutritional supplementation with transforming growth factor-beta, glutamine, and short chain fatty acids minimizes methotrexate-induced injury. J Pediatr Gastroenterol Nutr. 2006 Jan;42(1):53-8.
120. Yeh CL, Hsu CS, Yeh SL, Chen WJ. Dietary glutamine supplementation modulates Th1/Th2 cytokine and interleukin-6 expressions in septic mice. Cytokine. 2005 Sep 7;31(5):329-34.
121. Basivireddy J, Jacob M, Balasubramanian KA. Oral glutamine attenuates indomethacin-induced small intestinal damage. Clin Sci (Lond). 2004 Sep;107(3):281-9.
122. Li N, Liboni K, Fang MZ, Samuelson D, Lewis P, Patel R, Neu J. Glutamine decreases lipopolysaccharide-induced intestinal inflammation in infant rats. Am J Physiol Gastrointest Liver Physiol. 2004 Jun;286(6):G914-21.
123. Zhou X, Li YX, Li N, Li JS. Glutamine enhances the gut-trophic effect of growth hormone in rat after massive small bowel resection. J Surg Res. 2001 Jul;99(1):47-52. K60 RepairviteTM
124. Li N, Li J, Li Y. [Nutritional rehabilitation for patients with extreme short bowel]. Zhonghua Wai Ke Za Zhi. 1997 Dec;35(12):707-9.
125. Gismondo MR, Drago L, Fassina MC, Vaghi I, Abbiati R, Grossi E. Immunostimulating effect of oral glutamine. Dig Dis Sci. 1998 Aug;43(8):1752-4.
126. Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A, Yamamoto S. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997 Oct;41(4):487-93.
127. Elia M, Lunn PG. The use of glutamine in the treatment of gastrointestinal disorders in man. Nutrition. 1997 Jul-Aug;13(7-8):743-7.
128. Wu G, Meier SA, Knabe DA. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr. 1996 Oct;126(10):2578-84.
129. Marks IN. Site-protective agents. Baillieres Clin Gastroenterol. 1988 Jul;2(3):609-20. Review.
130. Marks IN, Boyd E. Mucosal protective agents in the long-term management of gastric ulcer. Med J Aust. 1985 Feb 4;142(3):S23-5.
131. Russell RI, Morgan RJ, Nelson LM. Studies on the protective effect of deglycyrrhinised liquorice against aspirin (ASA) and ASA plus bile acidinduced gastric mucosal damage, and ASA absorption in rats. Scand J Gastroenterol Suppl. 1984;92:97-100.
132. Bennett A. Gastric mucosal formation of prostanoids and the effects of drugs. Acta Physiol Hung. 1984;64(3-4):215-7.
133. Morgan RJ, Nelson LM, Russell RI, Docherty C. The protective effect of deglycyrrhinized liquorice against aspirin and aspirin plus bile acid-induced gastric mucosal damage, and its influence on aspirin absorption in rats. J Pharm Pharmacol. 1983 Sep;35(9):605-7.
134. Glick L. Deglycyrrhizinated liquorice for peptic ulcer. Lancet. 1982 Oct 9;2(8302):817.
135. van Marle J, Aarsen PN, Lind A, van Weeren-Kramer J. Deglycyrrhizinised liquorice (DGL) and the renewal of rat stomach epithelium. Eur J Pharmacol. 1981 Jun 19;72(2-3):219-25.
136. Datla R, Rao SR, Murthy KJ. Excretion studies of nitrofurantoin and nitrofurantoin with deglycyrrhizinated liquorice. Indian J Physiol Pharmacol. 1981 Jan-Mar;25(1):59-63.
137. Rees WD, Rhodes J, Wright JE, Stamford LF, Bennett A. Effect of deglycyrrhizinated liquorice on gastric mucosal damage by aspirin. Scand J Gastroenterol. 1979;14(5):605-7.
138. Balakrishnan V, Pillai MV, Raveendran PM, Nair CS. Deglycyrrhizinated liquorice in the treatment of chronic duodenal ulcer. J Assoc Physicians India. 1978 Sep;26(9):811-4.
139. D’Imperio N, Giuliani Piccari G, Sarti F, Soffritti M, Spongano P, Benvenuti C, Dal Monte PR. Double-blind trial in duodenal and gastric ulcers. Cimetidine and deglycyrrhizinized liquorice. Acta Gastroenterol Belg. 1978 Jul-Aug;41(7-8):427-34.
140. Bardhan KD, Cumberland DC, Dixon RA, Holdsworth CD. Proceedings: Deglycyrrhizinated liquorice in gastric ulcer: a double blind controlled study. Gut. 1976 May;17(5):397.
141. Larkworthy W, Holgate PF. Deglycyrrhizinized liquorice in the treatment of chronic duodenal ulcer. A retrospective endoscopic survey of 32 patients. Practitioner. 1975 Dec;215(1290):787-92.
142. Aarsen PN. Standardization method of deglycyrrhizinized liquorice on experimental gastric ulcers in rats. Arzneimittelforschung. 1973 Sep;23(9): 1346-8.
143. Engqvist A, von Feilitzen F, Pyk E, Reichard H. Double-blind trial of deglycyrrhizinated liquorice in gastric ulcer. Gut. 1973 Sep;14(9):711-5.
144. Håkanson R, Liedberg G, Oscarson J, Rehfeld JF, Stadil F. Effect of deglycyrrhizinized liquorice on gastric acid secretion, histidine decarboxylase activity and serum gastrin level in the rat. Experientia. 1973 May 15;29(5):570-1.
145. Whiting B, Thomson TJ. Deglycyrrhizinized liquorice in duodenal ulcer. Br Med J. 1971 Oct 2;4(5778):48.
146. Feldman H, Gilat T. A trial of deglycyrrhizinated liquorice in the treatment of duodenal ulcer. Gut. 1971 Jun;12(6):449-51.
147. Andersson S, Bárány F, Caboclo JL, Mizuno T. Protective action of deglycyrrhizinized liquorice on the occurrence of stomach ulcers in pylorus-ligated rats. Scand J Gastroenterol. 1971;6(8):683-6.
148. Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010 Apr;56(3):290-9.
149. Gunn-Moore DA, Shenoy CM. Oral glucosamine and the management of feline idiopathic cystitis. J Feline Med Surg. 2004 Aug;6(4):219-25.
150. Seidman EG, Bernotti S, Levy E. Nutritional modulation of gut inflammation. Nestle Nutr Workshop Ser Clin Perform Programme. 2002;7:41-61; discussion 61-5.
151. Sharma R, Schumacher U. Carbohydrate expression in the intestinal mucosa. Adv Anat Embryol Cell Biol. 2001;160:III-IX, 1-91.
152. Khan A, Ahmad A, Manzoor N, Khan LA. Antifungal activities of Ocimum sanctum essential oil and its lead molecules. Nat Prod Commun. 2010 Feb;5(2):345-9.
153. Fawole OA, Amoo SO, Ndhlala AR, Light ME, Finnie JF, Van Staden J. Anti-inflammatory, anticholinesterase, antioxidant and phytochemical properties of medicinal plants used for pain-related ailments in South Africa. J Ethnopharmacol. 2010 Feb 3;127(2):235-41.
154. Ozsoy N, Candoken E, Akev N. Implications for degenerative disorders: Antioxidative activity, total phenols, flavonoids, ascorbic acid, betacarotene and beta-tocopherol in Aloe vera. Oxid Med Cell Longev. 2009 Apr;2(2):99-106.
155. Kigondu EV, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, Kirira PG, Irungu B, Ingonga JM, Ndiege IO. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J Ethnopharmacol. 2009 Jun 25;123(3):504-9.
156. Chen W, Lu Z, Viljoen A, Hamman J. Intestinal drug transport enhancement by Aloe vera. Planta Med. 2009 May;75(6):587-95.
157. Pogribna M, Freeman JP, Paine D, Boudreau MD. Effect of Aloe vera whole leaf extract on short chain fatty acids production by Bacteroides fragilis, Bifidobacterium infantis and Eubacterium limosum. Lett Appl Microbiol. 2008 May;46(5):575-80.
158. Rosca-Casian O, Parvu M, Vlase L, Tamas M. Antifungal activity of Aloe vera leaves. Fitoterapia. 2007 Apr;78(3):219-22.
159. Iena IaM. [The therapeutic properties of aloe]. Lik Sprava. 1993 Feb-Mar;(2-3):142-5.
160. t’Hart LA, van den Berg AJ, Kuis L, van Dijk H, Labadie RP. An anticomplementary polysaccharide with immunological adjuvant activity from the leaf parenchyma gel of Aloe vera. Planta Med. 1989 Dec; 55(6):509-12.
161. Brossat JY, Ledeaut JY, Ralamboranto L, Rakotovao LH, Solar S, Gueguen A, Coulanges P. [Immunostimulating properties of an extract isolated from Aloe vahombe. 2. Protection in mice by fraction F1 against infections by Listeria monocytogenes, Yersinia pestis, Candida albicans
and Plasmodium berghei]. Arch Inst Pasteur Madagascar. 1981;48(1):11-34.
162. Delaporte RH, Sarragiotto MH, Takemura OS, Sánchez GM, Filho BP, Nakamura CV. Evaluation of the antioedematogenic, free radical scavenging and antimicrobial activities of aerial parts of Tillandsiastreptocarpa Baker-Bromeliaceae. J Ethnopharmacol. 2004 Dec;95(2-3):229-33.
163. de Queiroga MA, de Andrade LM, Florêncio KC, de Fátima Agra M, da Silva MS, Barbosa-Filho JM, da-Cunha EV. Chemical constituents from Tillandsia recurvata. Fitoterapia. 2004 Jun;75(3-4):423-5.
164. Arslanian RL, Stermitz FR, Castedo L. 3-Methoxy-5-hydroxyflavonols from Tillandsia purpurea. J Nat Prod. 1986 Nov-Dec;49(6):1177-8.
165. FEURT SD, FOX LE. Effects of oral administration of Spanish moss, Tillandsia usneoides L. Science. 1953 Nov 20;118(3073):626-7.
166. FEURT SD, FOX LE. The pharmacological activity of substances extracted from Spanish moss, Tillandsia usneoides L. J Am Pharm Assoc Am Pharm Assoc (Baltim). 1952 Aug;41(8):453-4.
167. WEBBER MG, LAUTER WM, FOOTE PA. A preliminary phytochemical study of Tillandsia usneoides L. (Spanish moss). J Am Pharm Assoc Am Pharm Assoc. 1952 May;41(5):230-5.
168. Hage-Sleiman R, Mroueh M, Daher CF. Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharm Biol. 2011 Mar;49(3):327-33.
169. Deters A, Zippel J, Hellenbrand N, Pappai D, Possemeyer C, Hensel A. Aqueous extracts and polysaccharides from Marshmallow roots (Althea officinalis L.): cellular internalisation and stimulation of cell physiology of human epithelial cells in vitro. J Ethnopharmacol. 2010 Jan 8;127(1):62-9.
170. Kardosová A, Machová E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia. 2006 Jul;77(5):367-73. Epub 2006 May 24.
171. Wang DF, Shang JY, Yu QH. [Analgesic and anti-inflammatory effects of the flower of Althaea rosea (L.) Cav.]. Zhongguo Zhong Yao Za Zhi. 1989 Jan;14(1):46-8, 64.
172. Iauk L, Lo Bue AM, Milazzo I, Rapisarda A, Blandino G. Antibacterial activity of medicinal plant extracts against periodontopathic bacteria. Phytother Res. 2003 Jun;17(6):599-604.
173. Satia JA, Littman A, Slatore CG, Galanko JA, White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol Biomarkers Prev. 2009 May;18(5):1419-28.
174. Marañón G, Muñoz-Escassi B, Manley W, García C, Cayado P, de la Muela MS, Olábarri B, León R, Vara E. The effect of methyl sulphonyl methane supplementation on biomarkers of oxidative stress in sport horses following jumping exercise. Acta Vet Scand. 2008 Nov 7;50:45.
175. Brien S, Prescott P, Bashir N, Lewith H, Lewith G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM)in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2008 Nov;16(11):1277-88.
176. Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002 Feb;7(1):22-44.
177. Maoka T, Tanimoto F, Sano M, Tsurukawa K, Tsuno T, Tsujiwaki S, Ishimaru K, Takii K. Effects of dietary supplementation of ferulic acid and gammaoryzanol on integument color and suppression of oxidative stress in cultured red sea bream, Pagrus major. J Oleo Sci. 2008;57(2):133-7.
178. Accinni R, Rosina M, Bamonti F, Della Noce C, Tonini A, Bernacchi F, Campolo J, Caruso R, Novembrino C, Ghersi L, Lonati S, Grossi S, Ippolito S, Lorenzano E, Ciani A, Gorini M. Effects of combined dietary supplementation on oxidative and inflammatory status in dyslipidemic subjects. Nutr Metab Cardiovasc Dis. 2006 Mar;16(2):121-7.
179. Sierra S, Lara-Villoslada F, Olivares M, Jiménez J, Boza J, Xaus J. Increased immune response in mice consuming rice bran oil. Eur J Nutr. 2005 Dec;44(8):509-16.
180. Jariwalla RJ. Rice-bran products: phytonutrients with potential applications in preventive and clinical medicine. Drugs Exp Clin Res. 2001;27(1):17-26.
181. Sugano M, Tsuji E. Rice bran oil and human health. Biomed Environ Sci. 1996 Sep;9(2-3):242-6.
182. Hirose M, Ozaki K, Takaba K, Fukushima S, Shirai T, Ito N. Modifying effects of the naturally occurring antioxidants gamma-oryzanol, phytic acid, tannic acid and n-tritriacontane-16, 18-dione in a rat wide-spectrum organ carcinogenesis model. Carcinogenesis. 1991 Oct;12(10):1917-21.
183. Wheeler KB, Garleb KA. Gamma oryzanol-plant sterol supplementation: metabolic, endocrine, and physiologic effects. Int J Sport Nutr. 1991 Jun;1(2):170-7.
184. Itaya K, Kitonaga J, Ishikawa M. [Studies of gamma-oryzanol. (2) The antiulcerogenic action]. Nippon Yakurigaku Zasshi. 1976 Nov;72(8):1001-11.
185. Brown AC, Hairfield M, Richards DG, McMillin DL, Mein EA, Nelson CD. Medical nutrition therapy as a potential complementary treatment for psoriasis-five case reports. Altern Med Rev. 2004 Sep;9(3):297-307.
186. Choi HR, Choi JS, Han YN, Bae SJ, Chung HY. Peroxynitrite scavenging activity of herb extracts. Phytother Res. 2002 Jun;16(4):364-7.
187. Majchrowicz MA. Essiac. Notes Undergr. 1995 Winter;(no 29):6-7.
188. Gill RE, Hirst EL, Jones JK. Constitution of the mucilage from the bark of Ulmus fulva (slippery elm mucilage); the sugars formed in the hydrolysis of the methylated mucilage. J Chem Soc. 1946 Nov:1025-9.
189. Jarrahi M, Vafaei AA, Taherian AA, Miladi H, Rashidi Pour A. Evaluation of topical Matricaria chamomilla extract activity on linear incisional wound healing in albino rats. Nat Prod Res. 2010 May;24(8):697-702.
190. Bezerra SB, Leal LK, Pinto NA, Campos AR. Bisabolol-induced gastroprotection against acute gastric lesions: role of prostaglandins, nitric oxide, and KATP+ channels. J Med Food. 2009 Dec;12(6):1403-6.
191. Moura Rocha NF, Venâncio ET, Moura BA, Gomes Silva MI, Aquino Neto MR, Vasconcelos Rios ER, de Sousa DP, Mendes Vasconcelos SM, de França Fonteles MM, de Sousa FC. Gastroprotection of (-)-alphabisabolol on acute gastric mucosal lesions in mice: the possible involved pharmacological mechanisms. Fundam Clin Pharmacol. 2009 Aug 3.
192. Pereira RP, Fachinetto R, de Souza Prestes A, Puntel RL, Santos da Silva GN, Heinzmann BM, Boschetti TK, Athayde ML, Bürger ME, Morel AF, Morsch VM, Rocha JB. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem Res. 2009 May;34(5):973-83.
193. Nogueira JC, Diniz Mde F, Lima EO. In vitro antimicrobial activity of plants in Acute Otitis Externa. Braz J Otorhinolaryngol. 2008 Jan-Feb;74(1):118-24.
194. Shikov AN, Pozharitskaya ON, Makarov VG, Kvetnaya AS. Antibacterial activity of Chamomilla recutita oil extract against Helicobacter pylori. Phytother Res. 2008 Feb;22(2):252-3.
195. Capasso R, Savino F, Capasso F. Effects of the herbal formulation ColiMil on upper gastrointestinal transit in mice in vivo. Phytother Res. 2007 Oct;21(10):999-1101.
196. Mahady GB, Pendland SL, Stoia A, Hamill FA, Fabricant D, Dietz BM, Chadwick LR. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother Res. 2005 Nov;19(11):988-91.
197. Madisch A, Holtmann G, Mayr G, Vinson B, Hotz J. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion. 2004;69(1):45-52.
198. Elango G, Rahuman AA, Kamaraj C, Zahir AA, Bagavan A. Studies on effects of indigenous plant extracts on filarial vector Culex tritaeniorhynchus Giles. Parasitol Res. 2010 Apr 7.
199. Aguilar HH, de Gives PM, Sánchez DO, Arellano ME, Hernández EL, Aroche UL, Valladares-Cisneros G. In vitro nematocidal activity of plant extracts of Mexican flora against Haemonchus contortus fourth larval stage. Ann N Y Acad Sci. 2008 Dec;1149:158-60.
200. Bashir S, Gilani AH. Studies on the antioxidant and analgesic activities of Aztec marigold (Tagetes erecta) flowers. Phytother Res. 2008 Dec;22(12):1692-4.
201. Wang M, Tsao R, Zhang S, Dong Z, Yang R, Gong J, Pei Y. Antioxidant activity, mutagenicity/ anti-mutagenicity, and clastogenicity/anticlastogenicity of lutein from marigold flowers. Food Chem Toxicol. 2006 Sep;44(9):1522-9.
202. Wang M, Tsao R, Zhang S, Dong Z, Yang R, Gong J, Pei Y. Antioxidant activity, mutagenicity/anti-mutagenicity, and clastogenicity/anticlastogenicity of lutein from marigold flowers. Food Chem Toxicol. 2006 Sep;44(9):1522-9.
203. Breithaupt DE, Bamedi A, Wirt U. Carotenol fatty acid esters: easy substrates for digestive enzymes? Comp Biochem Physiol B Biochem Mol Biol. 2002 Aug;132(4):721-8.
204. Garg SC, Dengre SL. Antibacterial activity of essential oil of Tagetes erecta Linn. Hindustan Antibiot Bull. 1986 Feb-Nov;28(1-4):27-9.
205. Martín-Venegas R, Brufau MT, Guerrero-Zamora AM, Mercier Y, Geraert PA, Ferrer R. The methionine precursor DL-2-hydroxy-(4-methylthio)butanoic acid protects intestinal epithelial barrier function. Food Chem. 2013 Dec 1;141(3):1702-9.
206. Flamant M, Aubert P, Rolli-Derkinderen M, Bourreille A, Neunlist MR, Mahé MM, Meurette G, Marteyn B, Savidge T, Galmiche JP, Sansonetti PJ, Neunlist M. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011 Apr;60(4):473-84.
207. van Ampting MT, Schonewille AJ, Vink C, Brummer RJ, van der Meer R, Bovee-Oudenhoven IM. Intestinal barrier function in response to abundant or depleted mucosal glutathione in Salmonella-infected rats. BMC Physiol. 2009 Apr 17:9:6
208. Maeda T, Miyazono Y, Ito K, Hamada K, Sekine S, Horie T. Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate treated rats. Cancer Chemother Pharmacol.2010 May;65(6):1117-2
209. Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007 Apr;132(4):1344-1358
210. The role of the glutathione antioxidant system in gut barrier failure in a rodent model of experimental necrotizing enterocolitis. Surgery. 2004 Sep;136(3):557-66
211. Mahmood A, FitzGerald AJ, Marchbank T, Ntatsaki E, Murray D, Ghosh S, Playford RJ.
Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. 2007 Feb;56(2):168-75. Epub 2006 Jun 15.
212. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastroint. Liver Physiol. 2008 Jan; 294(1):G208-16
213. Vassallo MF, Camargo CA Jr. Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. J Allergy Clin Immunol. 2010 Aug;126 (2):217-22
214. Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012 May 30;12:57.
215. Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol 1996;80:225–35.
216. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003;8:223–246.
217. Alla Skapenko, Jan Leipe, Peter E Lipsky, Hendrik Schulze-Koops The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005; 7(Suppl 2): S4–S14.
218. Jane M. Rodgers, Stephen D. Miller. Cytokine Control of Inflammation and Repair in the Pathology of Multiple Sclerosis. Yale J Biol Med. 2012 December; 85(4): 447–468.
219. Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998 Mar 17; 95(6):3071-6.
220. Murata Y, Shimamura T, Hamuro J. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int Immunol. 2002 Feb; 14(2):201-12.
221. Nazzareno Ballatori, Suzanne M. Krance, Sylvia Notenboom, Shujie Shi, Kim Tieu, Christine L. Hammond. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009 March; 390(3): 191–214.
222. Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Curr Med Chem. 2006:13(15): 1749-55
223. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing TH2 and TH17 cell development. Antioxid Redox Signal. 2010 Jun 6.
224. Venu Lagishetty, Nancy Q. Liu, Martin Hewison. Vitamin D metabolism and innate immunity.
Mol Cell Endocrinol. 2011 December 5; 347(1-2): 97–105.
225. Cynthia Aranow. Vitamin D and the Immune System. J Investig Med. 2011 August; 59(6): 881–886
226. Bansal AS, Henriquez F, Sumar N, Patel S. T helper cell subsets in arthritis and the benefits of immunomodulation by 1,25(OH)₂ vitamin D. Rheumatol Int. 2012 Apr;32(4):845-52.
227. Jot Hui Ooi, Jing Chen, Margherita T. Cantorna. Vitamin D regulation of immune function in the gut: Why do T cells have vitamin D receptors? Mol Aspects Med. 2012 February; 33(1): 77–82.
228. Diane L. Kamen, Vin Tangpricha. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 2010 May; 88(5): 441–450.
229. Margherita T. Cantorna. Vitamin D, Multiple Sclerosis and Inflammatory Bowel Disease.
Arch Biochem Biophys. 2012 July 1; 523(1): 103–106.
230. Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011 May 24;8:56
231. Danny Bruce, Jot Hui Ooi, Sanhong Yu, Margherita T Cantorna. Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med (Maywood) 2010 August; 235(8): 921–927.
232. Székely JI, Pataki Á. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: a short review. Expert Rev Respir Med. 2012 Dec;6(6):683-704. Review.
233. Ozkara S, Keles E, Ilhan N, Gungor H, Kaygusuz I, Alpay HC. The relationship between Th1/Th2 balance and 1α,25-dihydroxyvitamin D₃ in patients with nasal polyposis. Eur Arch Otorhinolaryngol. 2012 Dec;269(12):2519-24.
234. Taher YA, van Esch BCAM, Hofman GA, Henricks PAJ, van Oosterhout AJ. 1α,25-Dihydroxy-vitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-β1. Journal of Immunology. 2008;180(8):5211–5221
235. Székely JI, Pataki Á. Effects of vitamin D on immune disorders with special regard to asthma, COPD and autoimmune diseases: a short review. Expert Rev Respir Med. 2012 Dec;6(6):683-704. Review.
236. Maalmi H, Berraïes A, Tangour E, Ammar J, Abid H, Hamzaoui K, Hamzaoui A. The impact of vitamin D deficiency on immune T cells in asthmatic children: a case-control study. J Asthma Allergy. 2012;5:11-9.
237. Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: a comprehensive review. Clin Rev Allergy Immunol. 2013 Oct;45(2):217-26.
238. Gatenby P, Lucas R, Swaminathan A. Vitamin D deficiency and risk for rheumatic diseases: an update. Curr Opin Rheumatol. 2013 Mar;25(2):184-91. Review.
239. M I Koenders, W B van den Berg. Translational Mini-Review Series on Th17 Cells: Are T helper 17 cells really pathogenic in autoimmunity? Clin Exp Immunol. 2010 February; 159(2): 131–136.
240. Jesse M. Damsker, Anna M. Hansen, Rachel R. Caspi. Th1 and Th17 cells: Adversaries and collaborators. Ann N Y Acad Sci. 2010 January; 1183: 211–221.
241. Kiran Nistala, Stuart Adams, Helen Cambrook, Simona Ursu, Biagio Olivito, Wilco de Jager, Jamie G. Evans, Rolando Cimaz, Mona Bajaj-Elliott, Lucy R. Wedderburn. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010 August 17; 107(33): 14751–14756.
242. Jane M. Rodgers, Stephen D. Miller. Cytokine Control of Inflammation and Repair in the Pathology of Multiple Sclerosis. Yale J Biol Med. 2012 December; 85(4): 447–468.
243. Aristo Vojdani, Jama Lambert. The Role of Th17 in Neuroimmune Disorders: Target for CAM Therapy. Part I. Evid Based Complement Alternat Med. 2011; 2011: 927294.
244. Aristo Vojdani, Jama Lambert, Gottfried Kellermann. The Role of Th17 in Neuroimmune Disorders: A Target for CAM Therapy. Part III. Evid Based Complement Alternat Med. 2011; 2011: 548086.
245. Xie, L, Li XK, Takahara S. Curcumin has bright prospects for the treatment of multiple sclerosis. Int Immunopharmacol. 2011 Mar;11(3):323-330.
246. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Int Immunopharmacol. 2009 May;9(5):575-581
247. Petro TM. Regulatory role of resveratrol on Th17 in autoimmune disease. Int Immunopharmacol. 2011 Mar;11(3):310-318.
248. Aristo Vojdani, Jama Lambert, Gottfried Kellermann. The Role of Th17 in Neuroimmune Disorders: A Target for CAM Therapy. Part III. Evid Based Complement Alternat Med. 2011; 2011: 548086.
249. Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008a;453:106–109.
250. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71.
251. Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, Xu D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis 2012;71:129–135.
252. Koshland DE Jr. The molecule of the year. Science. 1992 Dec 18;258(5090):1861.
253. Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase
isoforms. Biochem Pharmacol. 1996;51(4):383-94.
254.Ulrich Förstermann, William C. Sessa Nitric oxide synthases: regulation and function. Eur Heart J. 2012 April; 33(7): 829–837.
255.Role of nitric oxide in inflammatory conditions. Nephron. 2002 Apr;90(4):373-8.
256.Regulatory role of nitric oxide on monocyte-derived dendritic cell functions. J Interferon Cytokine Res. 2003 Aug;23(8):423-31
257.Role of nitric oxide in the regulation of T-cell functions. Ann Rheum Dis. 2006 Nov;65 Suppl 3:iii37-40
258.Protective role of the cytokine-inducible isoform of nitric oxide synthase induction and nitrosative stress in experimental autoimmune encephalomyelitis of the DA rat. J Neurosci Res. 2003 Jul 15; 73(2):198-205
259.Immunoregulatory role of nitric oxide in Kilham rat virus-induced autoimmune diabetes in DR-BB rats. J Immunol. 2004 Jul 15;173(2):1327-35
260.Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 2004 Feb;15(1):21-32
261.Immunosuppressive and anti-inflammatory action of antioxidants in rat autoimmune diabetes. J Autoimmun. 2004 Jun;22(4):267-76
262.Agretesch HJ, Dagnelie PC, et al. Adensoine triphosphate, established and potential clinical
applications. Drugs. 1999;58:211-232.
263. Williams M, Bagwat SS. P2 purinoceptors: a family of novel therapeutic targets. Annual Reports in Medicinal Chemistry. 1996;31:21-30.
264. Haskell CM, Wong M, Williams A, and Leee LY. Phase 1 trial of extracellular adenosine
5’-triphosphate in patients with advanced cancer. Medicinal and Pediatric Oncology. 1996;27:165-173.
265.Mortensen DP, Gonzalez-Alonso J, Bune LT, et al. ATP-induced vasodilation and purinergic receptors in the human leg: roles of nitric oxide, prostaglandins and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140-R1148.
266.Yegutkin GG, Henttiner T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ectonucleotide kinase activities via phosphotransfer reactions. FASEB. 2001;15:251-260.
267.Persechini PM, Bisaggio RC, Alves Neto JL, Coutinho-Silva R. Extracellular ATP in the
lymphohematopoiectic system: P2Z purinoceptors and membrane permeabilization. Braz J of Med and Bio Res.1998;31(1):25-34.
268.Silva G, Beierwaltes WH, Garvin JL. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension.2006;47(3):563-7.
269.Tongguang W, Guangxiang H, Shuanke W, et al. Effects of extracellular ATP on survival of sensory neurons in the dorsal root ganglia of rats. J Tongi Med Univ. 2001;21(1):44-7.
270.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Cir Physiol. 1997;272:H1886-H1891.
271.Tonetti M, Sturia L, Bistolfi T, Benatti U, De Flora A. Extracellular ATP potentiates nitric oxide synthase expression induced by lipopolysaccharide in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 1994:203(1):430-5.
272. Li W, Mak M, Jian H, et al. Novel anti-Alzheimer’s dimmer Bis(7)-cognition: cellular and molecular mechanisms of neuroprotection through multiple targets. Neurotherapeutics. 2009: 6(1):187-201.
273.Li J , Wu HM, Zhou RL, Liu GJ, Dong BR. Huperzine A for Alzheimer’s disease. Cochrane
Database Sys Rev. 2008:16(2):CD005592.
274.Wang ZF, Tang XC. Huperzine A protects C6 rat glioma cells against oxygen-glucose deprivation –induced injury. FEBS Lett. 2007;581(4):596-602.
275.Zhao HW, Li XY. Ginkgolide A, B, and huperzine A inhibit nitric oxide-induced neurotoxicity. Int Immunopharmacol. 2002;2(11):1551-6.
276.Wang ZF, Tang XC. Huperzine A protects C6 rat glioma cells against oxygen-glucose deprivation–induced injury. FEBS Lett. 2007;581(4):596-602.
277.Pavlov VA, Parrish WR, Rosas-Ballina M. Brain acetylcholinesterase activity controls systemic levels through the cholinergic anti-infl ammatory pathway. Brain Behav Immun. 2009;23(1):41-5.
278.Wang ZF, Wang J, Zhang HY, Tang XC. Huperzine A exhibits anti-infl ammatory and neuroprotective effects in a rat model of transient focal cerebral ischemia. J Neurochem. 2008;106(4):1594-603.
279.Evgenov OV, Busch CJ, Evgenov NV, et al. Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake labs with acute pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L723-L729.
280.Mancina R, Filippi s, Marini M, et al. Expression and functional activity of phosphodies-terase type 5 in human and rabbit vas deferens. Mol Hum Reprod. 2005;11(2):107-115.
281.Oiu Y, Kraft P, Craig Ec, et al. Identifi cation and functional study of phosphodiesterases in rat urinary bladder. Urol Res. 2001;29(6):388-92.
282.Kim D, Rybalkin SD, Pi X. Upregualtion of phosphodiesterase 1A1 expression is associated with development of nitrate tolerance. Circulation. 2001. 104(19):2263-5.
283.Peyter AC, Muehlethaler V, Liaudet L. Muscarinic receptor M1 phosphodiesterase 1 are key determinants in pulmonary vascular dysfunction following perinatal hypoxia in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L201-213.
284.Kedia Gt, Ucker T S, Kedia M, Kuczyk MA. Effects of phosphodiesterase inhibitors on contraction induced by endothelin-1 of isolated human prostatic tissue. Urology.2009;73(6):1397-1401.
285.Souness Je, Brazdil R, Diocee BK, Jordan R.Role of selective cyclic GMP phosphodiesterase
inhibition in the myorelaxant actions of M&B 22, 948, MY-5455, vinopectine and 1-methyl-3-
isobutyl-8-(methylamino)xanthine. Br J Pharmacol. 1989. 98(3):725-34.
286.Lopez Cm, Govoni S, Battaini F, et al. Effect of a new cognition enhancer alphaglycerlphos-phorylcholine, on scopolamine induced amnesia and brain acetylcholine. Pharmacol Biochem Behav 1991;39(4): 835-40.
287.Sigala S, Imperato A , Rizzoneli P, et al. L-alpha glycerylphosphorylcholine antagonizes
scopolamine-induced amnesia and enhances hippocampal cholinergic transmission in the rat. Eur J Pharmacol 1992;211(3):351-8.
288.Sterin-Borda L, Echague AV, Leiros CP,et al. Endogenous nitrix oxide signaling system and the cardiac muscarinic acetylcholine receptor-inotropic response. Br J of Pharm. 1995;115:1525-1531.
289.Graves AR, Lewin KA, Lindgren CA. Nitrix oxide, camp and the biphasic muscarinic modulation of Ach release at the lizard neuromuscular junction. J Physiol. 2004;559.2:423-432.
290. Lindgren CA, Younk KM. The inhibition of Ach release by muscarinic agonists at the neuromuscular junction required nitric oxide synthesis. Soc Neurosci Abstr. 2002;28:838.3.
291.Lindgren CA, Graves AR, Lake B. Nitric oxide is necessary but not suffi cient for the biphasic muscarinic modulation of synaptic transmission at the lizard neuromuscular junction. Soc Neurosci Abstr. 2003:29:168.11
292.Zyad Rm, Qazi S, Morton DB, Trimmer, BA. Nicotinic-acetylcholine receptors are functionally coupled to the nitric oxide/cGMP-pathways in insect neruons. J neurochem. 2002;83(2):421-431.
293.Arvalho FA, Martine-Silva J, Mesquirea r, Salanha C. Acetylcholine and choline effects on
erthrocyte nitrite and nitrate levels. J Appl Tox.2004:24(6):419-427.
294.Robinson Le, Bussiere FL, Le Boucher J, et al. Amino acid nutrition and immune function in tumorbearing rats: a comparison of glutamine, arginine, and ornithine 2-oxoglutarate-supplmented diets. Clin Sci (Lond).1999:97(6):657-669.
295.Hammarqvist F, Wernerman J, von der Deckern A, Vinnars E. Alpha-ketoglutarate preserves protein synthesis and free glutamine in skeletal muscle after surgery. Surgery. 109(1):28-36.
296.Wernerman J, Hammarkvist F, Ali MR, Vinnars E. Glutamine and ornithine-alpha-ketogluterate but not branched-chain amino acids reduce the loss of muscle glutamine after surgical trauma. Metabolism. 1989:38(8 Suppl 1):63-6.
297.Wernerman J, Hammarqvist F, Vinnars E. Alpha-ketogluterate and postoperative muscle
catabolism. Lancet.1990:24;335(8691):701-3.
298.Seiwart H, Winterfeld HJ, Strangeld, et al. Effect of a 3-month interval running exercise in
combination with vasodilative therapy on microcirculation of the lower leg in peripheral arterial
circulation disorders (stage I and II). ZFA.1983;38(5):377-81.
299.Bieron K, Swies J, Kostka-Trabka E, Gryglewski RJ. Thrombolytic and anti-platelet action of xanithinol nicotinate (Sadmin):possible mechanisms.J Physiol Pharmacol.1998:49(2):241-9.
300.Sharma PL. Comparative effects of placebo and plain and slow-slow release tablets of
xanithinol nicotinate on exercise tolerance in normal subjects. Int J Clin Pharmacol Biopharm.
1978;16(1):19-21.
301.Starikov VI. The use of antioxidants and preparations improving the microcirculation in the surgical treatment of cancer of the lung in elderly patients. Klin Khir. 1991;10:4-6.
302.Gomez RD, Rojad HE. Therapeutic results with xanithine derivatives in the intermittent claudication syndrome in diabetic patients. Rev Clin Esp. 1988:182(1):3-6.
303.Janaki S. Pentoxifylline in strokes: a clinical study. J Int Med Res. 1980:8(1):56-62.
304.Mensen H. Rehabilitation course in patients with peripheral and coronary angioorganopathies. Studies with special reference to serum uric acid and cholesterol under xanthinol nicotinate. Ther Ggw. 1977:116(10):1853-81.
305.Spagnolie A, et al. Long-term acetyl-L-carnitine treatment in Alzheimer’s disease. Neurology 1991;41:1726-1732.
306.Bowman B. Acetylcholine and Alzheimer’s disease. Nutr Rev 1992; 50:142-144.
307.Careta A, et al. Acetyl-L-Carntine and Alzheimer’s disease. Pharmacological considerations
beyond cholinergic sphere. Ann NY Acad Sci 1993;695:324-326.
308.Pettegrew JW, et al. Clinical and neurochemical effects of acetyl-Lcarnitine in Alzheimer’s
disease. Neurobiol Aging 1995;16:1-4.
309.Sterin-Borda L, Echague AV, Leiros CP,et al. Endogenous nitrix oxide signaling system and the cardiac muscarinic acetylcholine receptor-inotropic response. Br J of Pharm. 1995;115:1525-1531.
310.Graves AR, Lewin KA, Lindgren CA. Nitrix oxide, camp and the biphasic muscarinic modulation of Ach release at the lizard neuromuscular junction. J Physiol. 2004;559.2:423-432.
311.Lindgren CA, Younk KM. The inhibition of Ach release by muscarinic agonists at the
neuromuscular junction required nitric oxide synthesis. Soc Neurosci Abstr. 2002;28:838.3.
312.Lindgren CA, Graves AR, Lake B. Nitric oxide is necessary but not suffi cient for the biphasic muscarinic modulation of synaptic transmission at the lizard neuromuscular junction. Soc Neurosci Abstr. 2003:29:168.11
313.Zyad Rm, Qazi S, Morton DB, Trimmer, BA. Nicotinic-acetylcholine receptors are functionally coupled to the nitric oxide/cGMP-pathways in insect neruons. J neurochem. 2002;83(2):421-431.
314.Arvalho FA, Martine-Silva J, Mesquirea r, Salanha C. Acetylcholine and choline effects on erthrocyte nitrite and nitrate levels. J Appl Tox.2004:24(6):419-427.
315.Himmerich H, Fischer J, Bauer K, Kirkby KC, Sack U, Krügel U. Stress-induced cytokine changes in rats. Eur Cytokine Netw. 2013 Jun;24(2):97-103.
316.Viktoriya Glovatchcka, Helena Ennes, Emeran A Mayer, Sylvie Bradesi. Chronic stress-induced changes in pro-inflammatory cytokines and spinal glia markers in the rat: a time course study. Neuroimmunomodulation. Author manuscript; available in PMC 2013 October 6.
317.Hannah Gola, Harald Engler, Annette Sommershof, Hannah Adenauer, Stephan Kolassa, Manfred Schedlowski, Marcus Groettrup, Thomas Elbert, Iris-Tatjana Kolassa. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013; 13: 40.
318.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: The brain and the immune system. Pharmacol Rev. 2000;52:595–638
319.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332:1351–1362.
320.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303.
321.Anisman H. Cascading effects of stressors and inflammatory immune system activation: Implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20.
322.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of cns pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59.
323.Maken DS, Weinberg J, Cool DR, Hennessy MB. An investigation of the effects of maternal separation and novelty on central mechanisms mediating pituitary-adrenal activity in infant guinea pigs (cavia porcellus). Behav Neurosci. 2011;124:800–809.
324.M. J. Finley, C. M. Happel, D. E. Kaminsky, T. J. Rogers. Opioid and Nociceptin Receptors Regulate Cytokine and Cytokine Receptor Expression. Cell Immunol. Author manuscript; available in PMC 2009 March 1.
325.Smith EM. Review Opioid peptides in immune cells. Adv Exp Med Biol. 2003; 521():51-68.
326.Carr DJ, Rogers TJ, Weber RJ. Review. The relevance of opioids and opioid receptors on immunocompetence and immune homeostasis. Proc Soc Exp Biol Med.1996 Dec; 213(3):248-57.
327.Walker JS. Anti-inflammatory effects of opioids. Adv Exp Med Biol. 2003;521:148-60.
328.Sacerdote P, Limiroli E, Gaspani L. Experimental evidence for immunomodulatory effects of opioids. Adv Exp Med Biol. 2003;521:106-16.
329.de Oliveira MS, da Silva Fernandes MJ, Scorza FA, Persike DS, Scorza CA, da Ponte JB, de Albuquerque M, Cavalheiro EA, Arida RM. Acute and chronic exercise modulates the expression of MOR opioid receptors in the hippocampal formation of rats. Brain Res Bull.2010 Oct 30;83(5):278-83.
330.Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician. 2012 Jul;15(3 Suppl):ES205-13. Review.
331.T A Howlett, S Tomlin, L Ngahfoong, L H Rees, B A Bullen, G S Skrinar, J W McArthur. Release of beta endorphin and met-enkephalin during exercise in normal women: response to training. Br Med J (Clin Res Ed) 1984 June 30; 288(6435): 1950–1952.
332.Harbach H, Hell K, Gramsch C, Katz N, Hempelmann G, Teschemacher H. Beta-endorphin (1-31) in the plasma of male volunteers undergoing physical exercise. Psychoneuroendocrinology. 2000 Aug;25(6):551-62.
333.Bulbulian R. Endogenous opioid effects on motoneuron pool excitability: potential analgesic effect of acute exercise. J Manipulative Physiol Ther. 2002 May;25(4):209-15.
334.Monnier L, Colette C. Glycemic variability: should we and can we prevent it? Diabetes Care. 2008 Feb;31 Suppl 2:S150-4. doi: 10.2337/dc08-s241.
335.Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008 Nov;2(6):1094-100.
336.Wysocka E, Cofta S, Piorunek T, Dziegielewska-Gesiak S, Bryl W, Torlinski L. Blood antioxidant status, dysglycemia and obstructive sleep apnea. Adv Exp Med Biol. 2013;756:121-9.
337.Standl E. Dysglycemia and abdominal obesity. Curr Vasc Pharmacol. 2012 Nov;10(6):678-9. Review.
338.Hovath EM, Benko R, Kiss L, Muranyi M, Pek T, Fekete K, et al. Rapid glycaemic swings induce oxidative stress, activate poly-(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009;52:952–61.
339.Maria Rosaria Rizzo, Michelangela Barbieri, Raffaele Marfella, Giuseppe Paolisso. Reduction of Oxidative Stress and Inflammation by Blunting Daily Acute Glucose Fluctuations in Patients With Type 2 Diabetes: Role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012 October; 35(10): 2076–2082.
340.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care. 2010 Jul;33(7):1529-35.
341.Razavi Nematollahi L, Kitabchi AE, Stentz FB, Wan JY, Larijani BA, Tehrani MM, Gozashti MH, Omidfar K, Taheri E. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009 Apr;58(4):443-8.
342.Dotson S, Freeman R, Failing HJ, Adler GK. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care. 2008 Jun;31(6):1222-3.
343.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care. 2010 Jul;33(7):1591-7.
344.Ling PR, Bistrian BR. Comparison of the effects of food versus protein restriction on selected nutritional and inflammatory markers in rats. Metabolism. 2009 Jun;58(6):835-42.
345.Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, Bucciarelli L, Rondinelli M, Genovese S. Vitamin C Further Improves the Protective Effect of Glucagon-like Peptide-1 on Acute Hypoglycemia-Induced Oxidative Stress, Inflammation, and Endothelial Dysfunction in Type 1 Diabetes. Diabetes Care. 2013 Oct 15.
346.Aristo Vojdani, Jama Lambert. The Role of Th17 in Neuroimmune Disorders: Target for CAM Therapy. Part I. Evid Based Complement Alternat Med. 2011; 2011: 927294.
347.Marietta EV, Murray JA. Animal models to study gluten sensitivity. Semin Immunopathol. 2012 Jul;34(4):497-511. Review.
348.Hernández-Lahoz C, Rodrigo L. [Gluten-related disorders and demyelinating diseases]. Med Clin (Barc). 2013 Apr 15;140(7):314-9. Review. Spanish.
349.Sabel’nikova EA.[Intolerance of gluten–a new disease or undiagnosed celiac disease]. Eksp Klin Gastroenterol. 2012;(3):87-9. Russian.
350.Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol. 2013 Sep;10(5):383-92.
351.Sanders DS, Aziz I. Non-celiac wheat sensitivity: separating the wheat from the chat! Am J Gastroenterol. 2012 Dec;107(12):1908-12
352.Nijeboer P, Mulder C, Bouma G. [Non-coeliac gluten sensitivity: hype, or new epidemic?]. Ned Tijdschr Geneeskd. 2013;157(21):A6168. Review. Dutch.
353.Guan WJ, Liu XJ, Tang BS, Liu YT, Zhou Y, Jiang H, Shen L, Xia K, Wang JL. Gluten ataxia of sporadic and hereditary cerebellar ataxia in patients from mainland China. Neurol India. 2013 May-Jun;61(3):226-30.
354.Fernandez-Jimenez N, Castellanos-Rubio A, Plaza-Izurieta L, Irastorza I, Elcoroaristizabal X, Jauregi-Miguel A, Lopez-Euba T, Tutau C, de Pancorbo MM, Vitoria JC, Bilbao JR. Coregulation and modulation of NFκB-related genes in celiac disease: uncovered aspects of gut mucosal inflammation. Hum Mol Genet. 2013 Oct 31.
355.Eric V. Marietta, Joseph A. Murray. Animal Models to Study Gluten Sensitivity. Semin Immunopathol. Author manuscript; available in PMC 2013 July 1.
356.Tanabe S. Analysis of food allergen structures and development of foods for allergic patients. Biosci Biotechnol Biochem. 2008;72:649–659. doi: 10.1271/bbb.70708.
357.Sapone A, Lammers KM, Mazzarella G, Mikhailenko I, Cartenì M, Casolaro V, Fasano A. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2010;152:75–80.
358.Molberg O, Uhlen AK, Jemsen T, Flaete NS, Fleckenstein B, Arentz-Hansen H, Raki M, Lundin KE, Sollid LM. Mapping of gluten T-cell epitopes in the bread wheat ancestors: implications for celiac disease. Gastroenterology. 2005;128:393–401. doi: 10.1053/j.gastro.2004.11.003.
359.Daniel DiGiacomo, Antonella Santonicola, Fabiana Zingone, Edoardo Troncone, Maria Cristina Caria, Patrizia Borgheresi, Gianpaolo Parrilli, Carolina Ciacci.Human leukocyte antigen DQ2/8 prevalence in non-celiac patients with gastrointestinal diseases. World J Gastroenterol. 2013 April 28; 19(16): 2507–2513.
360.Fine, Kenneth, “Early Diagnosis of Gluten Sensitivity: Before the Villi are Gone,” Celiac.com, http://www.celiac.com/articles/759/1/Early-Diagnosis-of-Gluten-Sensitivity-Before-the-Villi-are-Gone-By-Kenneth-Fine-MD/Page1.html.
361.Anna Sapone, Julio C Bai, Carolina Ciacci, Jernej Dolinsek, Peter HR Green, Marios Hadjivassiliou, Katri Kaukinen, Kamran Rostami, David S Sanders, Michael Schumann, Reiner Ullrich, Danilo Villalta, Umberto Volta, Carlo Catassi, Alessio Fasano. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012; 10: 13.
362.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175.
363.Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005 Sep;2(9): 416-22
364.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175.
365.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006 May;55(5):1443-9.
366. Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009 May;1165:195-205
367.Green P and Cellier C. Celiac Disease. N Engl J Med, 2007; 357:1731-1743.
368.Faulkner-Hogg KB, Selby WS, Loblay RH. Dietary analysis in symptomatic patients with Coeliac disease on a gluten-free diet: the role of trace amounts of gluten and non-gluten food intolerances. Scand J Gastroenterol, 1999; 34:384-389.
369.Sdepanian VL, Scaletsky ICA, Fagundes-Neto U, de Morais MB. Assessment of gliadin in supposedly gluten-free foods prepared and purchased by Celiac patients. J Ped Gastroenterol Nutr, 2001; 32:65-70.
370.Kristjánsson G, Venge P, Hällgren R. Mucosal reactivity to cow’s milk protein in Coeliac disease. Clin Exp Immunol. 2007; 147(3):449-455.
371.Eckman J, Saini SS, Hamilton RG. Diagnostic evaluation of food-related allergic diseases. Allergy Asthma ClinImmunol, 2009; 5(1):2.
372.Wildner G and Diedrichs-Möhring M. Autoimmune uveitis induced by molecular mimicry of peptides from rotavirus, bovine casein and retinal S-antigen. Eur J Immunol, 2003; 33:2577-2587.
373.Zanoni G, Navone R, Lunardi C, et al. In Celiac disease, a subset of autoantibodies against transglutaminase binds to toll-like receptor 4 and induces activation of monocytes. PLoS Med, 2006; 3(9):1637-1653.
374.Blutt SE, Crawford SE, Warfield KL, et al. The VP7 outer capsid protein of rotavirus induces polyclonal B-cell activation. J Virol, 2004; 78:6971-6981.
375.Sollid LM and Gray GM. A role for bacteria in Celiac disease? Am J Gastroenterol, 2004; 99:905-906.
376. Cabrera-Chávez F, Rouzaud-Sández O, Sotelo-Cruz N, et al. Bovine milk caseins and transglutaminase-treated cereal prolamins are differentially recognized by IgA of Celiac disease proteins according to their age. J Agric Food Chem, 2009; 57:3754-3759.
377. Becker CG, Van Hamont N, Wagner M. Tobacco, cocoa, coffee, and ragweed: cross-reacting allergens that activate factor-XII-dependent pathways. Blood, 1981; 58(5):861-867.
378.Vojdani A and Tarash I. Cross-reaction between gliadin and different food and tissue antigens. Food Nutri Sci, 2013; 4:20-32.
379.Scott H, Fausa O, Ek J, Brandtzaeg P. Immune response patterns in Coeliac disease. Serum antibodies to dietary antigens measured by an enzyme linked immunosorbent assay (ELISA). Clin Exp Immunol, 1984; 57(1):25-32.
380.Kristjánsson G, Venge P, Hällgren R. Mucosal reactivity to cow’s milk protein in Coeliac disease. Clin Exp Immunol. 2007; 147(3):449-455.
381.Cyrex Labs Array 4: Gluten-associated cross-reactive foods and foods sensitivity.™ www.cyrexlabs.com
- Hvatum M, Scott H, Brandtzaeg P. Serum IgG subclass antibodies to a variety of food antigens in patients with Coeliac disease. Gut, 1992; 33(5):632-638.
- Husby S, Foged N, Oxelius VA, Svehag SE. Serum IgG subclass antibodies to gliadin and other dietary antigens in children with Coeliac disease. Clin Exp Immunol, 1986; 64(3):526-35.
384. Gangur V, Kelly C, Navuluri L. Sesame allergy: a growing food allergy of global proportions? Ann Allergy Asthma Immunol, 2005; 95:4-11.
385.Popa V, Gavrilescu N, Preda N, et al. An investigation of allergy in byssinosis: sensitization to cotton, hemp, flax and jute antigens. Brit J Industr Med, 1969; 26:101-108.
386. Hekkens WT. The determination of prolamins in gluten-free food. Introductory remarks. Panminerva Med, 1991; 33(2):61-64.
387.Kim J-L, Wieslander G, Norbäck D. Allergy/Intolerance to buckwheat and other food products among Swedish subjects with Celiac disease. Proc. 9th Int’l Symp Buckwheat, Prague, 2004; 74:705-709.
388.Anna Sapone, Julio C Bai, Carolina Ciacci, Jernej Dolinsek, Peter HR Green, Marios Hadjivassiliou, Katri Kaukinen, Kamran Rostami, David S Sanders, Michael Schumann, Reiner Ullrich, Danilo Villalta, Umberto Volta, Carlo Catassi, Alessio Fasano. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012; 10: 13.

