Irritable Bowel Syndrome (IBS)
Irritable bowel syndrome (IBS) affects as many as 5%-20% of individuals worldwide and has a significant impact on quality of life. IBS symptoms range from diarrhea to constipation, or a combination of the two, with abdominal pain or discomfort and gas or bloating, referred to as “abdominal distension”. The degree of symptoms varies in different patients from tolerable to severe, and the time pattern and discomfort varies immensely from patient to patient. Some patients complain of daily symptoms, while others report intermittent symptoms at intervals of weeks or months.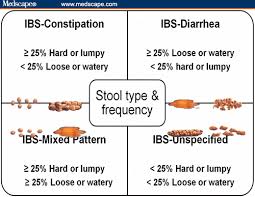 [360,361] There are no biomarkers used in the diagnosis of IBS so diagnosis is based on assessment of symptoms.[362,363] IBS is divided into subtypes, depending on symptoms such as diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C) and mixed diarrhea and constipation-predominant IBS (IBS-M).[364,365] IBS is not known to be associated with the development of serious disease or with excess mortality. However, IBS causes a reduced quality of life with the same degree of impairment as major chronic diseases.[366,367] The annual costs in the United States, both direct and indirect, for the management of patients with IBS are estimated at $15-30 billion.[368,369]
[360,361] There are no biomarkers used in the diagnosis of IBS so diagnosis is based on assessment of symptoms.[362,363] IBS is divided into subtypes, depending on symptoms such as diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C) and mixed diarrhea and constipation-predominant IBS (IBS-M).[364,365] IBS is not known to be associated with the development of serious disease or with excess mortality. However, IBS causes a reduced quality of life with the same degree of impairment as major chronic diseases.[366,367] The annual costs in the United States, both direct and indirect, for the management of patients with IBS are estimated at $15-30 billion.[368,369]
 What Causes IBS?
What Causes IBS?
The pathogenesis of IBS seems to be multifactorial, with the following factors most likely playing a central role in the pathogenesis of IBS: genetics, diet, gut flora, low-grade inflammation, and disturbances in the neuroendocrine system (NES) of the gut, which includes the enteric nervous system (ENS).[360,361] One hypothesis published recently in a worldwide gastroenterology journal 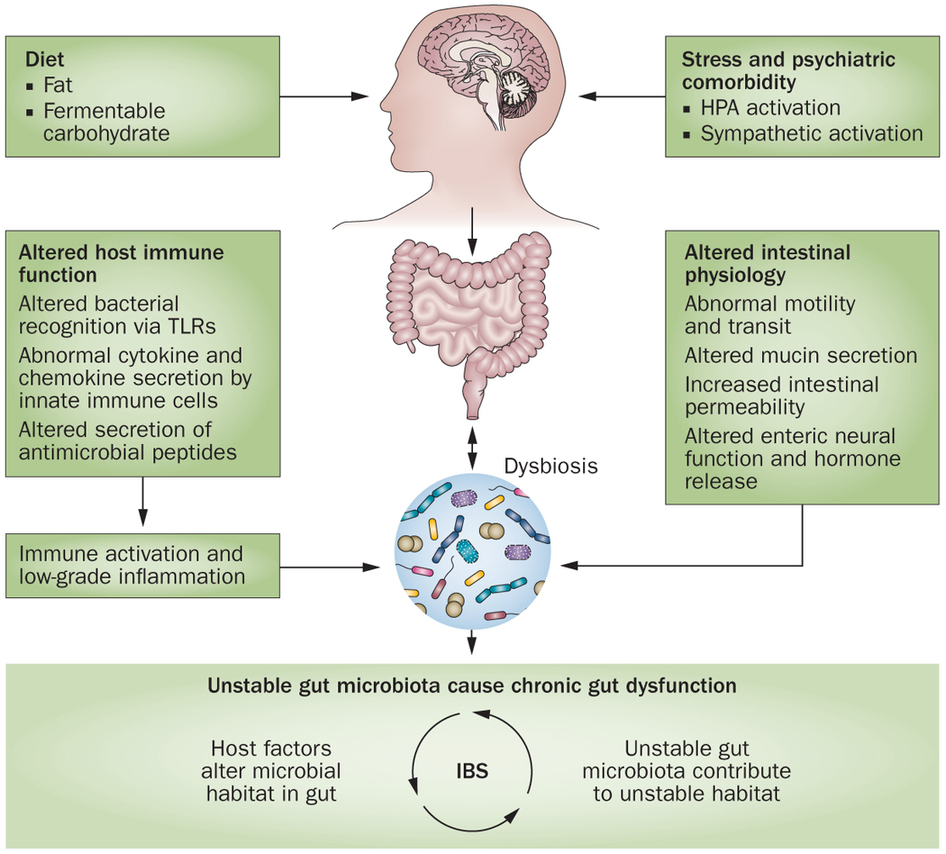 proposes that the cause of IBS is an altered NES of the gut, which accounts for abnormal GI motility, secretions and sensations.[360] All of these abnormalities are characteristic of IBS. The researchers propose that alterations in the NES could be the result of one or more of the following causative factors of IBS: genetic factors, diet, intestinal flora, or low-grade inflammation.[360] Let’s take a look at each of factors that are believed to contribute to the development of IBS.
proposes that the cause of IBS is an altered NES of the gut, which accounts for abnormal GI motility, secretions and sensations.[360] All of these abnormalities are characteristic of IBS. The researchers propose that alterations in the NES could be the result of one or more of the following causative factors of IBS: genetic factors, diet, intestinal flora, or low-grade inflammation.[360] Let’s take a look at each of factors that are believed to contribute to the development of IBS.
 Heritability and Genetics
Heritability and Genetics
Genetics appear to play a role in IBS. Up to 33% of patients with IBS had a family history of IBS compared to 2% of the controls in one study.[370] In another study of a family cluster, a significant association was reported between having a first degree family member with bowel symptoms and presenting with IBS. In contrast, those who reported having a spouse with bowel symptoms were no more likely to have IBS symptoms than the general population.[371] It was also shown that the prevalence of IBS was 17% in the relatives of patients compared to 7% in the relatives of spouses.[372] Another study showed that patients with IBS were more likely to present a family history of IBS than controls (33.9% and 12.6%, respectively).[373]
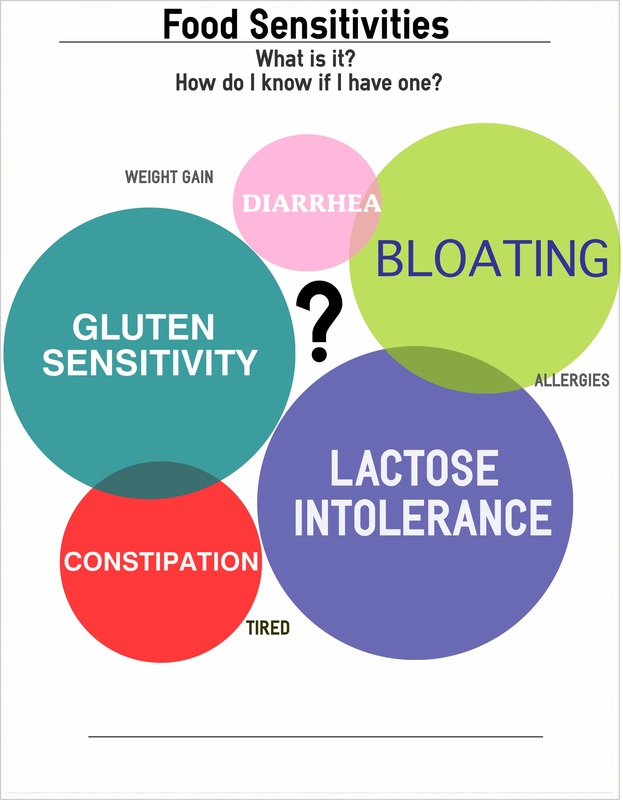 Does Diet Play a Role?
Does Diet Play a Role?
Most patients with IBS believe that their diet has a significant influence on their symptoms and they are interested in finding out which foods they should avoid.[374-377] There is some evidence supporting dietary influence on IBS symptoms. In one study, about 60% of IBS patients reported a worsening of symptoms following specific food ingestion: 28% within 15 min after eating and 93% within 3 hrs.[377] Many IBS patients report specific foods as triggers, most commonly implicating dairy and wheat products, but other foods as well such as onion, peas and beans, hot spices, cabbage, certain meats, smoked products, fried food and caffeine.[378]
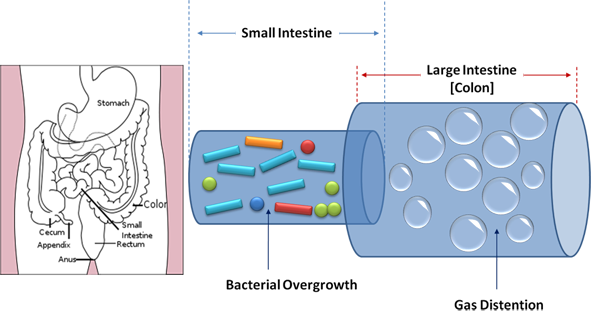
 The reaction of IBS patients to certain food items has been attributed to a number of short-chain carbohydrates called FODMAPs that are poorly digested and absorbed so that a significant portion of the ingested carbohydrates do not get digested properly and enter the small intestine and colon. Once there, these unabsorbed carbohydrates provide a substrate for bacterial fermentation with the production of gas and distension of the intestinal tract and an increase in intra-abdominal pressure (IAP). This leads to abdominal discomfort or pain. [360] Sound familiar? This is the same mechanism described earlier that lead to GERD and as we will see, also occurs in small intestinal bacterial overgrowth (SIBO).
The reaction of IBS patients to certain food items has been attributed to a number of short-chain carbohydrates called FODMAPs that are poorly digested and absorbed so that a significant portion of the ingested carbohydrates do not get digested properly and enter the small intestine and colon. Once there, these unabsorbed carbohydrates provide a substrate for bacterial fermentation with the production of gas and distension of the intestinal tract and an increase in intra-abdominal pressure (IAP). This leads to abdominal discomfort or pain. [360] Sound familiar? This is the same mechanism described earlier that lead to GERD and as we will see, also occurs in small intestinal bacterial overgrowth (SIBO).
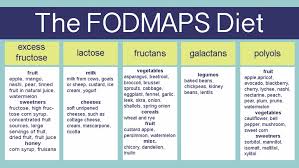 The FODMAPs Diet
The FODMAPs Diet
FODMAPs include fructose, lactose, fructans, galactans and sugar alcohols, such as sorbitol, maltitol, mannitol, xylitol and ismalt. The FODMAPs diet consists of excluding all these foods from the diet. Fructose and lactose are present in apples, pears, watermelon, honey, fruit juices, dried fruits, milk and dairy products. Polyols are used in low calorie food products. Galactans and fructans are present in common dietary food, such as wheat, rye, garlic, onions, legumes, cabbage, artichokes, leeks, asparagus, lentils, inulin, soy, brussel sprouts and broccoli.[379]
 Fiber
Fiber
A deficiency in dietary fiber was once widely believed to be the primary cause of IBS.[380] Although increasing the amount of dietary fiber continues to be a standard recommendation for patients with IBS, clinical practice has shown that increased fiber intake in these patients increases abdominal pain, bloating and distension.[380] In one study, IBS patients assigned to fiber treatment showed persistent symptoms or no improvement in symptoms after treatment compared to patients taking the placebo or a low-fiber diet but these patients were using common bran types of fiber.[380] It is noteworthy that the role of FODMAPs and fiber on IBS symptoms is associated with the intestinal flora. The presence of gut bacteria that break down FODMAPs and fiber and produce gas, such as Clostridia spp., can cause distension of the small and large intestine with abdominal discomfort or pain and lead to small intestinal bacterial overgrowth (SIBO).[381,382]
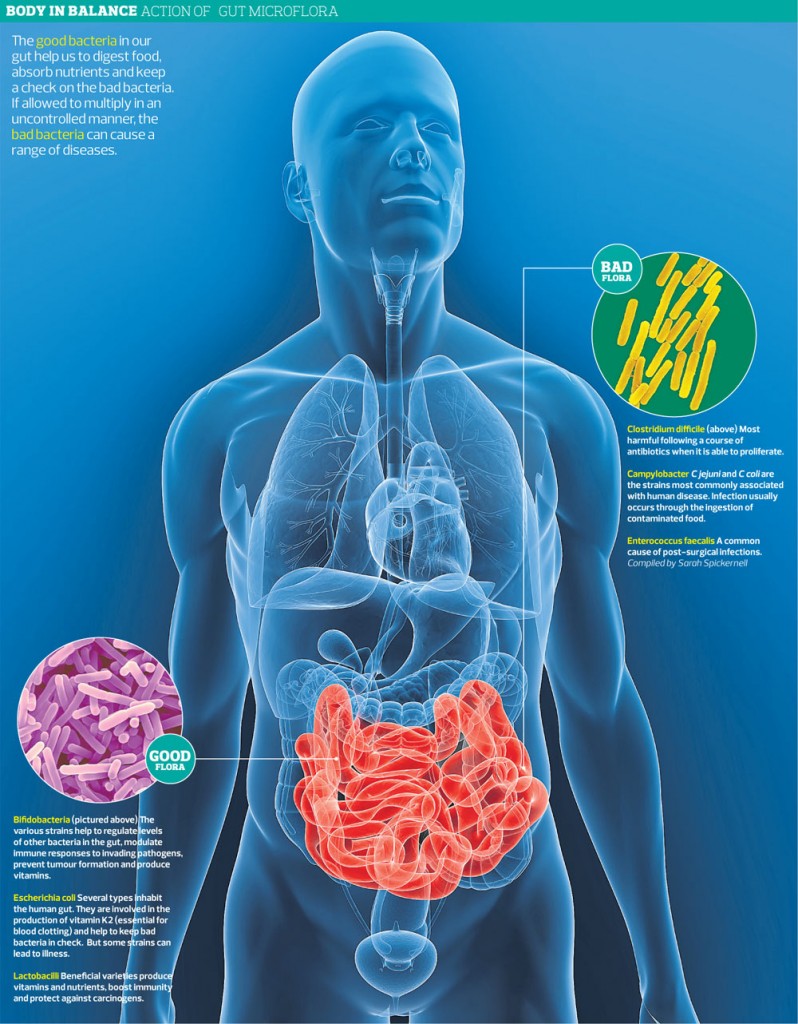 IBS and Intestinal Flora
IBS and Intestinal Flora
Most bacteria in the GI tract exist in the colon. The colon of each individual contains between 300 and 500 different species of bacteria, and each person has his own unique intestinal flora.[360] The intestinal flora is affected by several factors, such as diet, climate changes, stress, illness, aging and medications, such as acid-suppressing medication and antibiotic treatment.[383] The intestinal flora in IBS patients has been found to differ considerably from that of healthy controls, as IBS patients have fewer Lactobacillus and Bifidobacterium spp. than healthy subjects.[384] These bacteria bind to epithelial cells and inhibit pathogen binding as well as enhancing barrier functioning.[385] Furthermore, these bacterial species do not produce gas upon fermenting carbohydrates, which is an effect that would be amplified as they also inhibit the Clostridia spp.[385] Probiotics have been shown to decrease colonic fermentation and stabilize the colonic microbiota, and several studies on probiotics have shown improvements in flatulence and abdominal distension, with a reduction in the composite IBS symptom score.[385-387]
 A recent systematic review with meta-analysis published in a worldwide gastroenterology journal examined the efficacy of probiotics in the treatment of IBS. A total of 1,793 patients were included in the meta-analysis. Distension, bloating, and flatulence were evaluated using an IBS severity scoring system in three trials (two studies) to compare the effect of probiotic therapy in IBS patients with placebo. The review concluded the following:
A recent systematic review with meta-analysis published in a worldwide gastroenterology journal examined the efficacy of probiotics in the treatment of IBS. A total of 1,793 patients were included in the meta-analysis. Distension, bloating, and flatulence were evaluated using an IBS severity scoring system in three trials (two studies) to compare the effect of probiotic therapy in IBS patients with placebo. The review concluded the following:
“Probiotics reduce pain and symptom severity scores. The results demonstrate the beneficial effects of probiotics in IBS patients in comparison with placebo.”[388]
 IBS and Low-Grade Inflammation
IBS and Low-Grade Inflammation
Low-grade systemic inflammation appears to play a role in the development of IBS. In a subset of IBS patients, GI symptoms appear following gastroenteritis, with about 25% of patients showing diarrhea-predominant IBS (IBS-D) symptoms 6 months post- infection and approximately 10% developing persistent symptoms.[389-392] Post- infectious (PI)-IBS has been reported after viral, bacterial, protozoa and nematode infections[383], with the incidence of PI-IBS varying between 7% and 31%.[389-392] One study showed that 6% to 17% of sporadic IBS patients believed that their symptoms began with an infection.[383] Following infection, the initial inflammatory response shows an increase in certain types of lymphocytes (white blood cells) and macrophages.[389] These changes rapidly decrease in most subjects but a small number of patients with persistent symptoms continue to have signs of infection.[393]
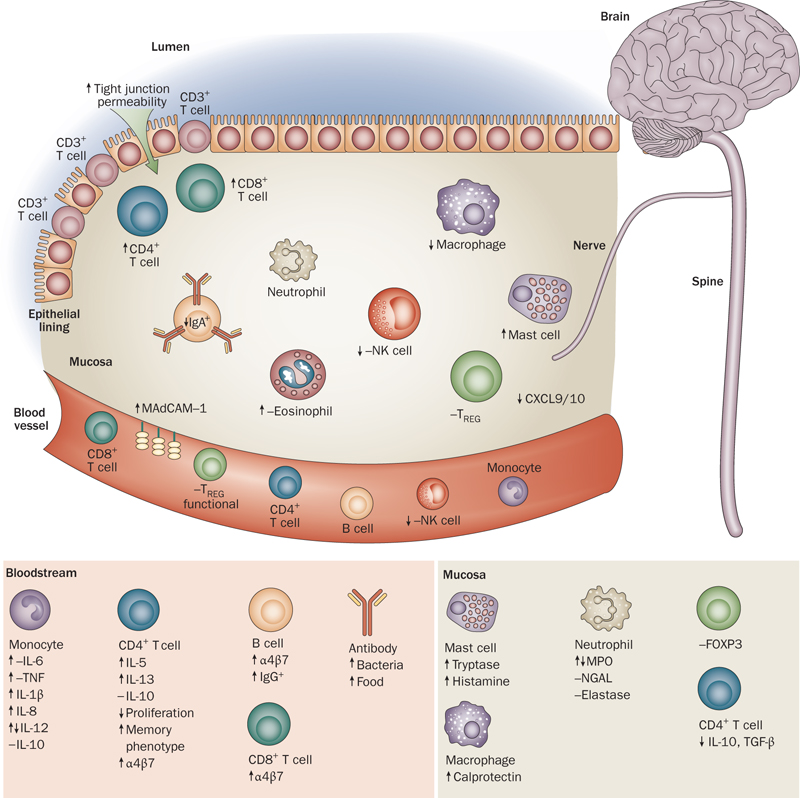 There are several pieces of evidence showing that inflammation and immune cells affect the neuroendocrine system (NES) of the gut, which controls and regulates GI motility and sensitivity. [394] There is also evidence of IBS patients having increased intestinal permeability and gut barrier issues than in the non-IBS population.[395-396]
There are several pieces of evidence showing that inflammation and immune cells affect the neuroendocrine system (NES) of the gut, which controls and regulates GI motility and sensitivity. [394] There is also evidence of IBS patients having increased intestinal permeability and gut barrier issues than in the non-IBS population.[395-396]
For a more in-depth perspective on the neuroendocrine system (NES) of the gut and the brain-gut axis and how abnormalities of the NES and the brain-gut axis may contribute to IBS, click on: The Neuroendocrine System of the Gut and the Brain-Gut Axis
 Is SIBO the Culprit?
Is SIBO the Culprit?
Some researchers now believe that irritable bowel syndrome (IBS) may be caused by bacterial overgrowth in the small intestine (SIBO).[487] An article was published in JAMA in 2004 proposing that small intestinal bacterial overgrowth (SIBO) might explain the symptoms of altered gut motility, visceral hypersensitivity, abnormal brain-gut interaction and immune activation seen in IBS.[487] This is supported by multiple lines of evidence. First, SIBO is found in up to 78%-84% of IBS patients undergoing lactulose breath testing indicating bacterial overgrowth.[488,489] Second, the distribution of inflammatory mediators and/or inflammatory cells have been shown to be disturbed in a percentage of patients with IBS.[490]
It is thought that SIBO may contribute to many of the clinical manifestations of IBS through bacterial fermentation and stimulation of a gut immune response, characterized by release of inflammatory mediators (such as interleukins and tumour necrosis factor-α) which may affect motility, secretion and sensation.[487,491] Additionally, postinfectious IBS, which occurs in 4%-31% of patients after an episode of acute gastroenteritis[492], also supports a causative role of bacteria in IBS.[493]
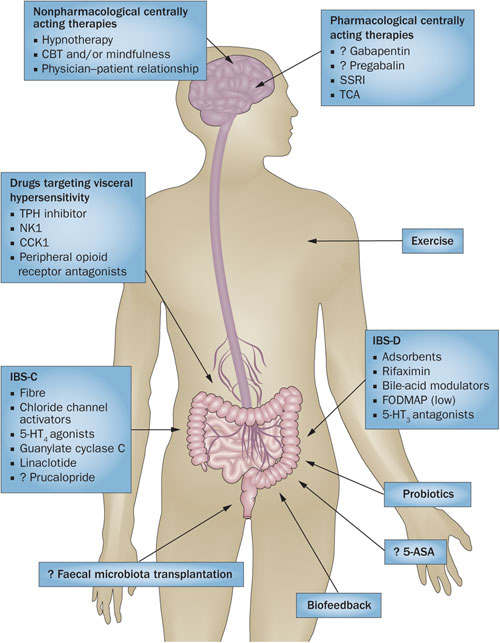 Conventional Treatment of IBS
Conventional Treatment of IBS
“A wide range of medications (prokinetics, antispasmodics, sedatives, tranquilizers, laxatives, fecal bulking agents, probiotics and antibiotics) along with lifestyle and diet modifications have been proposed for this highly prevalent condition; however to date there is no definite effective cure for this state.”[494]
Conventional treatment options for IBS include pharmacological drugs aimed at symptomatic relief of the primary symptoms involved in IBS, namely pain, diarrhea and constipation.[494] In addition, antibiotic drugs such as rifaximin are recently being used to treat IBS due to evidence of bacterial overgrowth in this condition.[495] Treatment for abdominal pain include antispasmodics, which encompass several different drug classes (smooth-muscle relaxants, antimuscarinics, anticholinergics) and other agents (pinaverium, trimebutine). 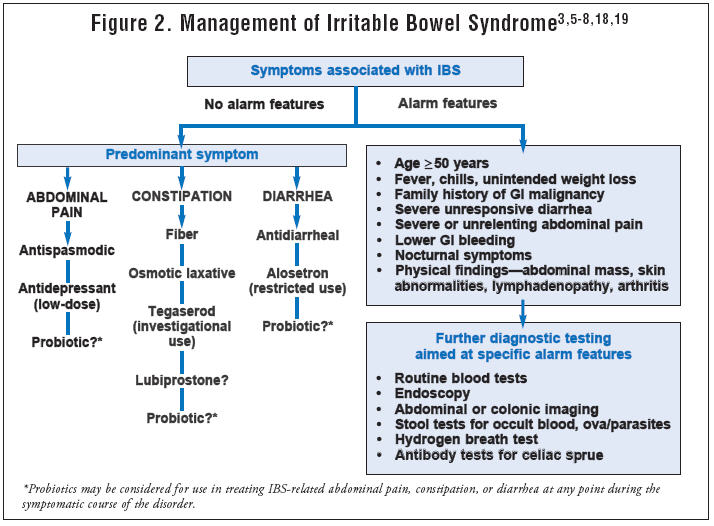 The anticholinergic properties of these agents restrict their usefulness in clinical practice.[494] Other drugs used for abdominal pain include opioid receptor agonists (exogenous opioids), GLP-1 (glucagon-like peptide 1) and membrane stabilizers (ketotifen). Membrane stabilizers act by blocking mast cell degranulation.[496] The number of mucosal mast cells has been studied and found positively correlated to abdominal pain.[497]
The anticholinergic properties of these agents restrict their usefulness in clinical practice.[494] Other drugs used for abdominal pain include opioid receptor agonists (exogenous opioids), GLP-1 (glucagon-like peptide 1) and membrane stabilizers (ketotifen). Membrane stabilizers act by blocking mast cell degranulation.[496] The number of mucosal mast cells has been studied and found positively correlated to abdominal pain.[497]
 Treatment for diarrhea-predominant IBS (IBS-D) include anti-diarrheals (loperamide), 5-HT3 antagonists or serotonin-blockers (alosetron, cilansetron, ramosetron), anti-inflammatory drugs (mesalamine) and antibiotic drugs (rifaximin). The potential use of antibiotics in IBS treatment has been supported by a growing body of evidence showing the important role that intestinal bacterial overgrowth plays in the development of IBS. Various lines of evidence suggest that small intestinal bacterial overgrowth (SIBO) may lead to many of the symptoms seen in IBS, such as altered gut motility, visceral hypersensitivity, abnormal brain-gut interaction and immune activation seen in IBS.[494,498] The American Task Force systematic review of IBS treatments concludes that rifaximin has shown improvement of global IBS symptoms and bloating in trials included in their analysis.[499]
Treatment for diarrhea-predominant IBS (IBS-D) include anti-diarrheals (loperamide), 5-HT3 antagonists or serotonin-blockers (alosetron, cilansetron, ramosetron), anti-inflammatory drugs (mesalamine) and antibiotic drugs (rifaximin). The potential use of antibiotics in IBS treatment has been supported by a growing body of evidence showing the important role that intestinal bacterial overgrowth plays in the development of IBS. Various lines of evidence suggest that small intestinal bacterial overgrowth (SIBO) may lead to many of the symptoms seen in IBS, such as altered gut motility, visceral hypersensitivity, abnormal brain-gut interaction and immune activation seen in IBS.[494,498] The American Task Force systematic review of IBS treatments concludes that rifaximin has shown improvement of global IBS symptoms and bloating in trials included in their analysis.[499]
 Treatment for constipation-predominant IBS (IBS-C) include laxative-based treatments (osmotic agents, and stool softeners), prokinetics (drugs to stimulate movement of bowels), prosecretory agents (drugs that augment intestinal secretion, thus acting as a stool lubricant and facilitating its evacuation, such as lubiprostone and linaclotide), serotonin-boosting drugs (serotonin receptor modulators and 5-HT4 agonists such as tegaserod) and bile acid modulators (CDC and elobixibat). Bile acid modulators have been used to treat constipation disorders based on the observation of increased incidence of diarrhea in patients taking bile acids for gallstones or cholestatic liver diseases.[500]
Treatment for constipation-predominant IBS (IBS-C) include laxative-based treatments (osmotic agents, and stool softeners), prokinetics (drugs to stimulate movement of bowels), prosecretory agents (drugs that augment intestinal secretion, thus acting as a stool lubricant and facilitating its evacuation, such as lubiprostone and linaclotide), serotonin-boosting drugs (serotonin receptor modulators and 5-HT4 agonists such as tegaserod) and bile acid modulators (CDC and elobixibat). Bile acid modulators have been used to treat constipation disorders based on the observation of increased incidence of diarrhea in patients taking bile acids for gallstones or cholestatic liver diseases.[500]
 However, these drugs have limited effects in their use due to their symptomatic approach and risk of various side effects.
However, these drugs have limited effects in their use due to their symptomatic approach and risk of various side effects.
“Pharmacologic treatments for IBS-C have largely been unsatisfactory, mainly due to the multifaceted and poorly understood pathophysiology of this disorder.”[501]
“The multifactorial pathogenesis of the disease and the ill-defined drug targets make the goal of manufacturing a “universal drug” for IBS-C a hard one to attain.”[500]
 Evidence of the long-term benefit of pharmacological treatment has been sparse, and new drugs that have proven to be effective have raised issues concerning safety.[502,503] These agents have limited usefulness as most of them are directed toward the symptoms of IBS, rather than the underlying causes of this condition, with the possible exception of antibiotic therapy. Alternative therapies, such as cognitive behavioral therapy and gut-directed hypnotherapy, have been used with good results.[504] A more efficient approach would be to address the underlying factors that appear to be related to the development of IBS, such as excess carbohydrate consumption, food sensitivities, insufficient HCl and pancreatic enzyme production, balanced intestinal flora, low-grade intestinal inflammation and the brain-gut axis. This is exactly how we approach IBS in the functional medicine model.
Evidence of the long-term benefit of pharmacological treatment has been sparse, and new drugs that have proven to be effective have raised issues concerning safety.[502,503] These agents have limited usefulness as most of them are directed toward the symptoms of IBS, rather than the underlying causes of this condition, with the possible exception of antibiotic therapy. Alternative therapies, such as cognitive behavioral therapy and gut-directed hypnotherapy, have been used with good results.[504] A more efficient approach would be to address the underlying factors that appear to be related to the development of IBS, such as excess carbohydrate consumption, food sensitivities, insufficient HCl and pancreatic enzyme production, balanced intestinal flora, low-grade intestinal inflammation and the brain-gut axis. This is exactly how we approach IBS in the functional medicine model.
 Clinical Management of IBS in Functional Medicine
Clinical Management of IBS in Functional Medicine
Clinical management of IBS in the functional medicine model starts with a thorough evaluation of the gut. This may include a comprehensive stool analysis in addition to a stool microbiology assay, assessing for dysbiosis and ruling out pathogenic bacterial, fungal or parasitic infection. A comprehensive stool analysis can provide a great deal of information about gut function including markers of protein and fat digestion, pancreatic function, and production of short-chain fatty acids such as butyric acid which are important for colon health.  A stool analysis can also assess for inflammatory gut conditions such as inflammatory bowel disease (ie: ulcerative colitis and Crohn’s disease) without undergoing intestinal biopsy and rule out GI bleeding. Assessment of related gut conditions, such as intestinal permeability and small intestinal bacterial overgrowth should be considered in conditions involving gas and bloating. Routine blood chemistry is usually done to assess inflammatory markers and assess other organ function that impact the gut such as thyroid function. Food sensitivity testing and nutritional analysis to assess for nutrient deficiencies should be considered as well.
A stool analysis can also assess for inflammatory gut conditions such as inflammatory bowel disease (ie: ulcerative colitis and Crohn’s disease) without undergoing intestinal biopsy and rule out GI bleeding. Assessment of related gut conditions, such as intestinal permeability and small intestinal bacterial overgrowth should be considered in conditions involving gas and bloating. Routine blood chemistry is usually done to assess inflammatory markers and assess other organ function that impact the gut such as thyroid function. Food sensitivity testing and nutritional analysis to assess for nutrient deficiencies should be considered as well.
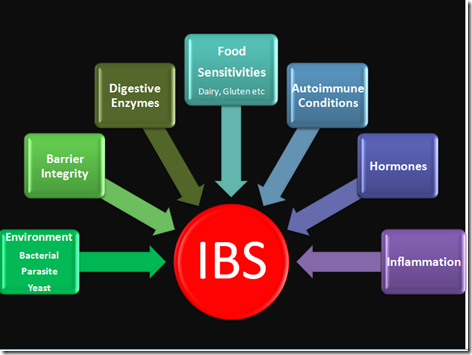 Management of IBS can vary greatly from person to person depending on the findings of the stool analysis as well as other conditions that may impact stomach and GI health such as intestinal permeability and food sensitivity. Treatment goals in the functional medicine model are to eliminate pathogenic infection, restore balance to the gut flora, and restore normal function of the stomach and GI tract (normal secretion of HCl and pancreatic enzymes, normal digestion of food and normal motility/movement of the intestines). This can normally be attained through the use of herbal anti-microbial compounds, probiotics and digestive enzyme therapy. Assessment of related organ function (particularly the thyroid, immune system, liver and brain) should be conducted to assess for imbalances in these organ systems as well.
Management of IBS can vary greatly from person to person depending on the findings of the stool analysis as well as other conditions that may impact stomach and GI health such as intestinal permeability and food sensitivity. Treatment goals in the functional medicine model are to eliminate pathogenic infection, restore balance to the gut flora, and restore normal function of the stomach and GI tract (normal secretion of HCl and pancreatic enzymes, normal digestion of food and normal motility/movement of the intestines). This can normally be attained through the use of herbal anti-microbial compounds, probiotics and digestive enzyme therapy. Assessment of related organ function (particularly the thyroid, immune system, liver and brain) should be conducted to assess for imbalances in these organ systems as well.
 Most IBS patients suffer from some degree of dysbiosis, so the use of probiotics and prebiotics are often employed to restore gut flora balance. Several specific bacterial probiotic strains have been shown to improve symptom severity and abdominal pain in IBS patients in numerous studies.[462,466-479] There are some species that in clinical trials appear to be more effective than others, such as Bifidobacterium species (B. infantis 3564 and B. bifidum MIMBb75) [470,474,476] and Lactobacillus species (Lactobacillus acidophilus-SDC 2012, 2013, L. paracasei B2106, L. plantarum 299V, and L. rhamnosus GG).[469,473,475] These probiotics appear to be effective in reducing abdominal pain and discomfort in adults and in children. Several studies have also suggested that combinations of different probiotic strains, such as VSL3# or mixtures of Bifidobacterium and Lactobacillus species, are able to decrease abdominal pain and discomfort in patients with IBS.[466,478,479]
Most IBS patients suffer from some degree of dysbiosis, so the use of probiotics and prebiotics are often employed to restore gut flora balance. Several specific bacterial probiotic strains have been shown to improve symptom severity and abdominal pain in IBS patients in numerous studies.[462,466-479] There are some species that in clinical trials appear to be more effective than others, such as Bifidobacterium species (B. infantis 3564 and B. bifidum MIMBb75) [470,474,476] and Lactobacillus species (Lactobacillus acidophilus-SDC 2012, 2013, L. paracasei B2106, L. plantarum 299V, and L. rhamnosus GG).[469,473,475] These probiotics appear to be effective in reducing abdominal pain and discomfort in adults and in children. Several studies have also suggested that combinations of different probiotic strains, such as VSL3# or mixtures of Bifidobacterium and Lactobacillus species, are able to decrease abdominal pain and discomfort in patients with IBS.[466,478,479]
 Addressing low-grade inflammation and intestinal permeability through gut-restorative and anti-inflammatory botanicals as well as dietary intervention is also important in IBS. There are a wide variety of compounds shown to repair the damaged intestinal lining, improve gut barrier integrity and decrease low-grade inflammation in the GI tract as described in the Intestinal Permeability section above.[288-351]
Addressing low-grade inflammation and intestinal permeability through gut-restorative and anti-inflammatory botanicals as well as dietary intervention is also important in IBS. There are a wide variety of compounds shown to repair the damaged intestinal lining, improve gut barrier integrity and decrease low-grade inflammation in the GI tract as described in the Intestinal Permeability section above.[288-351]
 Dietary compounds can induce immune responses in the GI tract and contribute to low-grade inflammation, particularly in gut permeability issues, therefore food sensitivity testing can be of value in IBS patients. This involves assessing for levels of IgA and IgG antibodies to various foods in the blood through a lab that offers specialized food sensitivity testing. Elimination of these foods from the diet is important when trying to repair a leaky gut or improve IBS symptoms. Lastly, assessing for small intestinal bacterial overgrowth (SIBO) and implementing a specific-carbohydrate diet (SCD) and/or low FODMAP diet in these cases is also important to reduce bacterial fermentation causing gas, bloating, abdominal discomfort and improve bowel regularity. We will learn more about the assessment of SIBO and how this condition is managed in the next section.
Dietary compounds can induce immune responses in the GI tract and contribute to low-grade inflammation, particularly in gut permeability issues, therefore food sensitivity testing can be of value in IBS patients. This involves assessing for levels of IgA and IgG antibodies to various foods in the blood through a lab that offers specialized food sensitivity testing. Elimination of these foods from the diet is important when trying to repair a leaky gut or improve IBS symptoms. Lastly, assessing for small intestinal bacterial overgrowth (SIBO) and implementing a specific-carbohydrate diet (SCD) and/or low FODMAP diet in these cases is also important to reduce bacterial fermentation causing gas, bloating, abdominal discomfort and improve bowel regularity. We will learn more about the assessment of SIBO and how this condition is managed in the next section.
Related Articles
Hypochlorhydria (Low Stomach Acid)
Gastroesophageal Reflux Disease (GERD)
Intestinal Permeability/Leaky Gut
Small Intestinal Bacterial Overgrowth
The Neuroendocrine System of the Gut and the Brain-Gut Axis
References
360. El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014 Jan 14;20(2):384-400. doi: 10.3748/wjg.v20. i2.384. Review.
361. Magdy El-Salhy. Irritable bowel syndrome: Diagnosis and pathogenesis. World J Gastroenterol. 2012 October 7; 18(37): 5151–5163. Published online 2012 October 7. doi: 10.3748/wjg.v18.i37.5151
362. Barbara G, Stanghellini V. Biomarkers in IBS: when will they replace symptoms for diagnosis and management? Gut. 2009;58:1571–1575. [PubMed]
363. Spiller RC. Potential biomarkers. Gastroenterol Clin North Am. 2011;40:121–139. [PubMed]
364. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. [PubMed]
365. Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, Jones R, Kumar D, Rubin G, Trudgill N, et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. 2007;56:1770–1798. [PMC free article] [PubMed]
366. Whitehead WE, Burnett CK, Cook EW, Taub E. Impact of irritable bowel syndrome on quality of life. Dig Dis Sci. 1996;41:2248–2253. [PubMed]
367. Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–660. [PubMed]
368. Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. [PubMed]
369. Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. [PubMed]
370. Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986;27:37–40. [PMC free article] [PubMed]
371. Locke GR, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ. Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;75:907–912. [PubMed]
372. Kalantar JS, Locke GR, Zinsmeister AR, Beighley CM, Talley NJ. Familial aggregation of irritable bowel syndrome: a prospective study. Gut. 2003;52:1703–1707. [PMC free article] [PubMed]
373. Kanazawa M, Endo Y, Whitehead WE, Kano M, Hongo M, Fukudo S. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig Dis Sci. 2004;49:1046–1053. [PubMed]
374. Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204–1214. [PubMed]
375. Morcos A, Dinan T, Quigley EM. Irritable bowel syndrome: role of food in pathogenesis and management. J Dig Dis. 2009;10:237–246. [PubMed]
376. Austin GL, Dalton CB, Hu Y, Morris CB, Hankins J, Weinland SR, Westman EC, Yancy WS, Drossman DA. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:706–708.e1. [PMC free article] [PubMed]
377. Simrén M, Månsson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, Björnsson ES. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–115.[PubMed]
378. Nanda R, James R, Smith H, Dudley CR, Jewell DP. Food intolerance and the irritable bowel syndrome. Gut. 1989;30:1099–1104. [PMC free article] [PubMed]
379. Austin GL, Dalton CB, Hu Y, Morris CB, Hankins J, Weinland SR, Westman EC, Yancy WS, Drossman DA. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:706–708.e1. [PMC free article] [PubMed]
380. Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. [PMC free article] [PubMed]
381. Andrew C. Dukowicz, Brian E. Lacy, Gary M. Levine. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterology Hepatol (NY). 2007 Feb;3(2):112-122.
382. Amit H. Sachdev, Mark Pimentel. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis. 2013. September; 4(5):223–231. doi:10.1177/ 2040622313496126
383. El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome. New York: Nova scientific Publisher; 2012.
384. Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. [PubMed]
385. Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:385–396. [PubMed]
386. Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009; 104:1033–1049; quiz 1050.[PubMed]
387. Levy RL, Linde JA, Feld KA, Crowell MD, Jeffery RW. The association of gastrointestinal symptoms with weight, diet, and exercise in weight-loss program participants. Clin Gastroenterol Hepatol. 2005;3:992–996. [PubMed]
388. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. 2015 Mar 14;21(10):3072-84. doi: 10.3748/wjg.v21.i10.3072.
389. Spiller RC. Role of infection in irritable bowel syndrome. J Gastroenterol. 2007;42 Suppl 17:41–47.[PubMed]
390. Spiller R, Garsed K. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis. 2009;41:844–849. [PubMed]
391. Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009; 136:1979–1988.[PubMed]
392. Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ. 1997;314:779–782. [PMC free article] [PubMed]
393. Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permea-bility following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47: 804–811.[PMC free article] [PubMed]
394. Spiller R. Serotonin, inflammation, and IBS: fitting the jigsaw together? J Pediatr Gastroenterol Nutr. 2007;45 Suppl 2:S115–S119. [PubMed]
395. Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol. 2011 Aug 11; [Epub ahead of print] [PMC free article] [PubMed]
396. Shulman RJ, Eakin MN, Czyzewski DI, et al. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr. 2008;153:646–50. [PMC free article] [PubMed]
487. Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. [PubMed]
488. Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506. [PubMed]
489. Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. [PubMed]
490. O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. [PubMed]
491. Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2007;25:1271–1281. [PubMed]
492. Connor BA. Sequelae of traveler’s diarrhea: focus on postinfectious irritable bowel syndrome. Clin Infect Dis. 2005;41 Suppl 8:S577–S586. [PubMed]
493. Lazaraki G, Chatzimavroudis G, Katsinelos P. Recent advances in pharmacological treatment of irritable bowel syndrome. World J Gastroenterol. 2014 Jul 21;20(27):8867-85. doi: 10.3748/wjg.v20.i27.8867. Review.
494. Lazaraki G, Chatzimavroudis G, Katsinelos P. Recent advances in pharmacological treatment of irritable bowel syndrome. World J Gastroenterol. 2014 Jul 21;20(27):8867-85. doi: 10.3748/wjg.v20.i27.8867. Review.
495. Laterza L, Ianiro G, Scoleri I, et al. Rifaximin for the treatment of diarrhoea-predominant irritable bowel syndrome. Expert Opin Pharmacother. 2015 Mar;16(4):607-15. doi: 10.1517/14656566.2015.1007951. Epub 2015 Feb 1.
496. Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. [PubMed]
497. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. [PubMed]
498. Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. JAMA. 2004;292:852–858. [PubMed]
499. Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–S35. [PubMed]
500. Iser JH, Sali A. Chenodeoxycholic acid: a review of its pharmacological properties and therapeutic use. Drugs. 1981;21:90–119. [PubMed]
501. Jadallah KA, Kullab SM, Sanders DS. Constipation-predominant irritable bowel syndrome: a review of current and emerging drug therapies. World J Gastroenterol. 2014 Jul 21;20(27):8898-909. doi: 10.3748/wjg.v20.i27.8898. Review.
502. Pasricha PJ. Desperately seeking serotonin… A commentary on the withdrawal of tegaserod and the state of drug development for functional and motility disorders. Gastroenterology. 2007;132:2287–2290.[PubMed]
503. Wald A, Rakel D. Behavioral and complementary approaches for the treatment of irritable bowel syndrome. Nutr Clin Pract. 2008;23:284–292. [PubMed]
504. Schmulson MJ, Ortiz-Garrido OM, Hinojosa C, Arcila D. A single session of reassurance can acutely improve the self-perception of impairment in patients with IBS. J Psychosom Res. 2006;61:461–467.[PubMed]
