Intestinal Permeability/ Leaky Gut
In this section we will take a hard look at the most common disorders of the intestinal tract, with a focus on the physiologic mechanisms that lead to these disorders and how they can be corrected with natural compounds and dietary changes. We will start this section by examining the literature on a topic that is gaining a lot of attention in gastroenterology research as well as the field of immunology: intestinal permeability or “leaky gut”. Let’s start by explaining exactly what this condition refers to.
 What is Leaky Gut?
What is Leaky Gut?
The lining of the gastrointestinal (GI) tract is composed of small epithelial cells that lie side-by-side each other forming tight junctions. These tight junctions act as a barrier between the interior of the body (blood/circulatory system) and the exterior of the body (the lumen of the GI tract). There are two primary functions of the epithelial lining of the GI tract: the first is to absorb small micromolecules, such as digested food particles, which are used as fuel sources and other processes for the body. These micronutrients are absorbed directly into the epithelial cells or between the cells and enter the bloodstream after passing through the gut-associated lymphoid tissue (GALT), the underlying intestinal immune system. The GALT serves as a containment system, preventing potentially harmful antigens from reaching systemic circulation.
 “A more attentive analysis of the anatomic and functional arrangement of the gastrointestinal tract, however, suggests that another extremely important function of this organ is its ability to regulate the trafficking of macromolecules between the environment and the host through a barrier mechanism.”[275]
“A more attentive analysis of the anatomic and functional arrangement of the gastrointestinal tract, however, suggests that another extremely important function of this organ is its ability to regulate the trafficking of macromolecules between the environment and the host through a barrier mechanism.”[275]
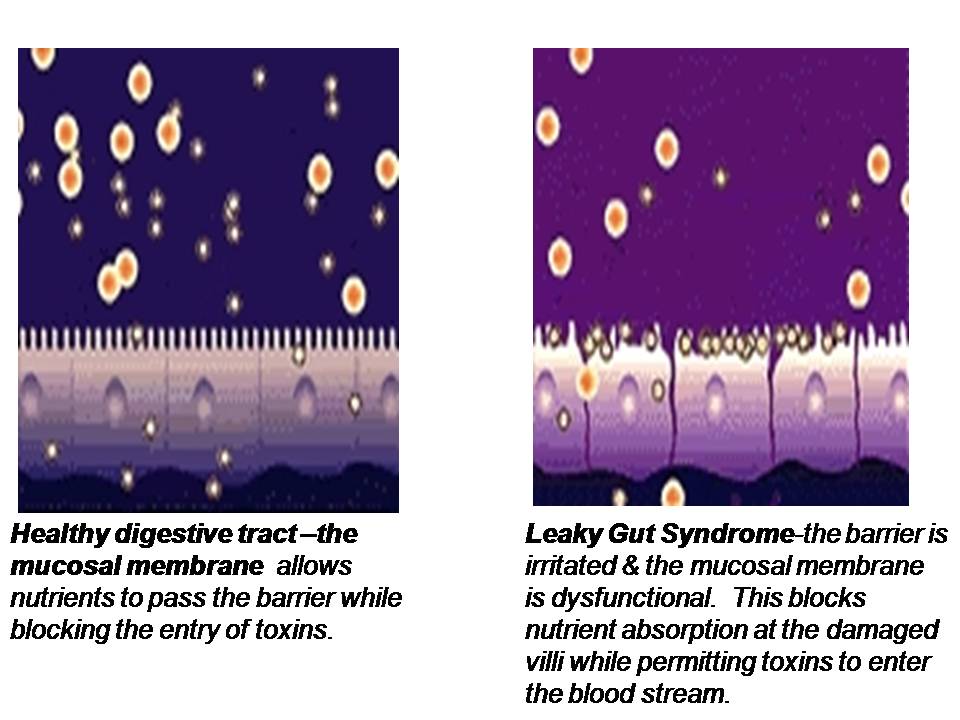
The second function of the epithelial lining of the GI tract, is to act as a barrier and prevent the absorption of larger macromolecules which should not normally penetrate through the intestinal barrier. When the tight junctions (TJs) of the intestinal mucosa are compromised, they become widened and permeable to large undigested food compounds, toxins and bacteria. This is known as intestinal permeability or “leaky gut”. The larger compounds of absorbed yet undigested proteins are reacted against by the immune system of the gut, called the GALT (gut-associated lymphoid tissue). This reaction then promotes exaggerated immune responsiveness and intestinal inflammation.
 Think of the cells of your intestinal lining, where absorption of micromolecules takes place, as bricks which sit side by side along the basement membrane. These “bricks” are held together by proteins that form the tight junctions (TJs), which act as the “mortar” between the bricks. Micronutrients are absorbed into the epithelial cells and transported through the cells and basement membrane where they enter the bloodstream. If the “mortar” starts to break down, you get penetration of foreign molecules through the intestinal barrier which can then enter the bloodstream (hence the term “leaky gut”).
Think of the cells of your intestinal lining, where absorption of micromolecules takes place, as bricks which sit side by side along the basement membrane. These “bricks” are held together by proteins that form the tight junctions (TJs), which act as the “mortar” between the bricks. Micronutrients are absorbed into the epithelial cells and transported through the cells and basement membrane where they enter the bloodstream. If the “mortar” starts to break down, you get penetration of foreign molecules through the intestinal barrier which can then enter the bloodstream (hence the term “leaky gut”).
Sometimes the epithelial cells, or “bricks” themselves, start to break down allowing passage of toxins through the cells (transcellular passage) into the blood. These foreign molecules may include undigested food molecules, toxins or bacteria.
Intestinal Permeability Leads to Systemic Inflammation
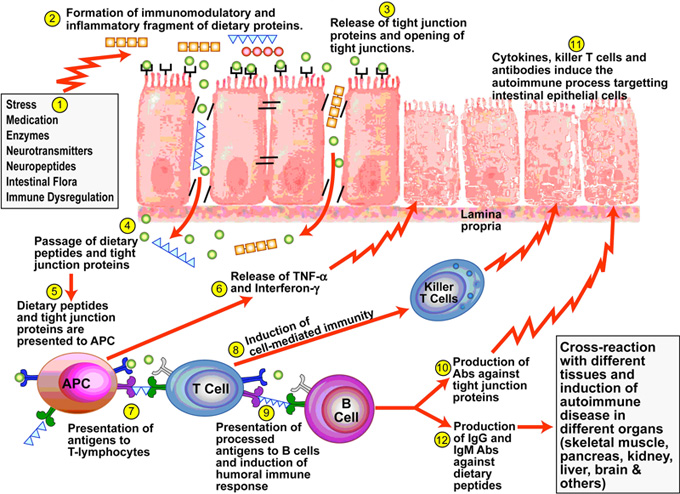 If anything penetrates through this gastrointestinal barrier that is foreign, the immune system is activated to eliminate or destroy these unwelcome molecules. This activation of the immune system leads to an inflammatory response. If you look at the anatomy of the intestinal lining, it is mostly immune cells with a thin layer of epithelial cells protecting it. If that epithelial layer gets breached, there is no question there will be an inflammatory immune response. So it is no surprise to see systemic inflammation and risk of autoimmunity when there is breach of this thin epithelial lining.
If anything penetrates through this gastrointestinal barrier that is foreign, the immune system is activated to eliminate or destroy these unwelcome molecules. This activation of the immune system leads to an inflammatory response. If you look at the anatomy of the intestinal lining, it is mostly immune cells with a thin layer of epithelial cells protecting it. If that epithelial layer gets breached, there is no question there will be an inflammatory immune response. So it is no surprise to see systemic inflammation and risk of autoimmunity when there is breach of this thin epithelial lining.
Increases in inflammatory responses increase risk of all chronic disease, including depression and neurodegenerative diseases, cardiovascular disease, bone loss, autoimmune disease, inflammatory bowel disease and chronic pain. A person’s intestinal barrier integrity will influence their inflammatory load and their potential for deteriorating mechanisms throughout the body.
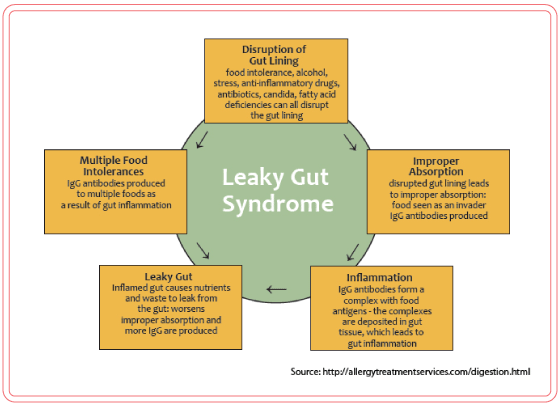 Health Conditions Associated with Leaky Gut Syndrome
Health Conditions Associated with Leaky Gut Syndrome
The following conditions have been associated with leaky gut syndrome:
- Autoimmune disorders
- Multiple food sensitivities
- Multiple chemical sensitivities
- Chronic fatigue syndrome
- Heart failure
- Depression
- Chronic inflammatory conditions
- Chronic pain
- Inflammatory bowel disease (IBD)
- Chronic yeast overgrowth syndromes
- Brain fog
 Intestinal Permeability and Autoimmune Disease
Intestinal Permeability and Autoimmune Disease
There is a growing amount of evidence establishing the connection between intestinal permeability and the development of autoimmune disease. This review paper was published in 2009 in the prestigious Annals of New York Academy of Science. This journal is one of the top-rated most powerful scientific journals available:
“There is growing evidence that increased intestinal permeability plays a pathogenic role in various autoimmune diseases. Therefore, we hypothesize that loss of intestinal barrier function is necessary to develop autoimmunity.”[276]
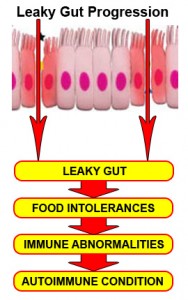 Basically, what this paper is saying, is that the research is pointing to intestinal permeability, or leaky gut, as a precondition for the development of all autoimmune disease, such as type I diabetes, rheumatoid arthritis (RA), lupus (SLE), multiple sclerosis (MS), Lou Gehrig’s disease (ALS), Hashimoto’s hypothyroidism, Grave’s disease, Crohn’s disease, ulcerative colitis, Celiac disease, and hundreds of other diseases.
Basically, what this paper is saying, is that the research is pointing to intestinal permeability, or leaky gut, as a precondition for the development of all autoimmune disease, such as type I diabetes, rheumatoid arthritis (RA), lupus (SLE), multiple sclerosis (MS), Lou Gehrig’s disease (ALS), Hashimoto’s hypothyroidism, Grave’s disease, Crohn’s disease, ulcerative colitis, Celiac disease, and hundreds of other diseases.
Here’s another paper supporting this discovery:
“Together with the gut-associated lymphoid tissue and the neuroendocrine network, the intestinal epithelial barrier, with its intercellular tight junctions, controls the equilibrium between tolerance and immunity to non-self antigens. When the finely-tuned trafficking of macromolecules is dysregulated in individuals, both intestinal and extraintestinal autoimmune disorders can occur.”[275]
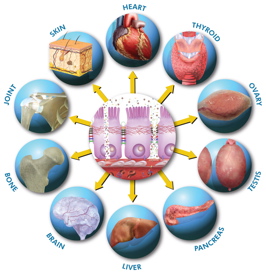 One of the hallmarks of a healthy immune system is immune tolerance to self-tissue. This prevents our immune system from attacking one’s own tissue of the body. We are born with an immune system that is able to differentiate self-tissue from foreign pathogens and does not attack self-tissue under normal circumstances. One of the things the research is showing is once these tight junction proteins get compromised, there is such immune zealousness and activation, this whole immune self-tolerance is lost and the immune system is prone to attacking self-tissue. When this occurs, any tissue is up for grabs for autoimmune destruction. The type of tissue that is targeted for destruction determines the type of autoimmune disease that develops.
One of the hallmarks of a healthy immune system is immune tolerance to self-tissue. This prevents our immune system from attacking one’s own tissue of the body. We are born with an immune system that is able to differentiate self-tissue from foreign pathogens and does not attack self-tissue under normal circumstances. One of the things the research is showing is once these tight junction proteins get compromised, there is such immune zealousness and activation, this whole immune self-tolerance is lost and the immune system is prone to attacking self-tissue. When this occurs, any tissue is up for grabs for autoimmune destruction. The type of tissue that is targeted for destruction determines the type of autoimmune disease that develops.
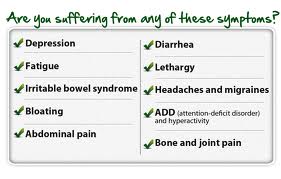
Symptoms of Intestinal Permeability
 Symptoms of intestinal permeability vary significantly and have a varied presentation. There is a spectrum of severity of intestinal permeability from mild to severe forms. Here is a list of the most common symptoms associated with intestinal permeability:
Symptoms of intestinal permeability vary significantly and have a varied presentation. There is a spectrum of severity of intestinal permeability from mild to severe forms. Here is a list of the most common symptoms associated with intestinal permeability:
- Bloating
- Poor Digestion
- Inflammation
- Food Sensitivities
- Chronic Pain
- Brain Fog/Poor Memory
- Depression
- Fatigue
 People with intestinal permeability will often react to certain types of foods, especially common food allergens such as wheat and dairy. Gluten sensitivity is very common with these people. Digestive abnormalities such as gas, bloating and food sensitivities are common symptoms of leaky gut. On the other side of the scale are people who never have intestinal symptoms but have other symptoms of chronic inflammation such as chronic pain, depression, brain fog or fatigue. They are too tired or in too much pain to go to the gym and exercise on a regular basis. When we see patients that present with these symptoms, we have to consider intestinal permeability issues.
People with intestinal permeability will often react to certain types of foods, especially common food allergens such as wheat and dairy. Gluten sensitivity is very common with these people. Digestive abnormalities such as gas, bloating and food sensitivities are common symptoms of leaky gut. On the other side of the scale are people who never have intestinal symptoms but have other symptoms of chronic inflammation such as chronic pain, depression, brain fog or fatigue. They are too tired or in too much pain to go to the gym and exercise on a regular basis. When we see patients that present with these symptoms, we have to consider intestinal permeability issues.
 Common Imbalances Associated with Leaky Gut
Common Imbalances Associated with Leaky Gut
There are a number of common imbalances that often occur together with leaky gut. These include:
- Malabsorption/malnutrition
- Bacterial/yeast overgrowth/dysbiosis (imbalance of beneficial and pathogenic bacteria)
- Intestinal lining degeneration/impaired intestinal immune integrity
- Immune activation/immune dysregulation/autoimmunity
- Food sensitivities
 Mechanisms Leading to Leaky Gut
Mechanisms Leading to Leaky Gut
There are many different environmental and dietary factors that may damage the epithelial lining of the GI tract, including a diet high in refined sugar, intake of food allergens, such as wheat and dairy, alcohol use, use of medications such as antibiotics or NSAIDs, such as ibuprofen and aspirin, stress, chronic infections, nutrient deficiencies, hormonal deficiencies (thyroid, estrogen or testosterone) and metabolic disturbances, such as diabetes or pre-diabetes.[277-286] All of these factors have been shown to contribute to intestinal permeability.
Alterations in intestinal physiology may affect the integrity of the intestinal epithelium tight junction protein structures. These mechanisms basically include physiological shifts that induce greater ongoing catabolism (breakdown) versus anabolism (buildup) of the epithelial mucosa. Additionally, systemic inflammation, which occurs in autoimmune conditions, promotes leaky gut. So this becomes a vicious cycle of leaky gut, leading to systemic inflammation, leading to further leaky gut.
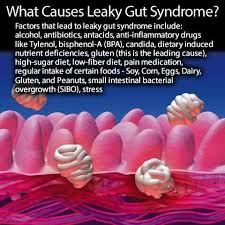 What are the Causes of Leaky Gut?
What are the Causes of Leaky Gut?
- Diet (refined sugar, wheat, dairy, etc.)
- Medication (antibiotics, NSAIDs, etc.)
- Infection (bacterial, viral, parasitic)
- Stress
- Hormonal Imbalances (thyroid, estrogen, testosterone, etc.)
- Neurologic Issues (stroke, brain injury, etc.)
- Metabolic Issues (diabetes, pre-diabetes, etc.)
- Autoimmunity
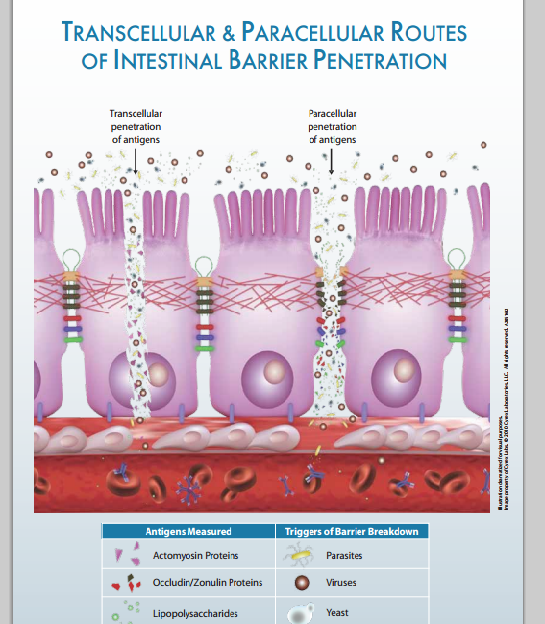
Assessment of Intestinal Permeability: Intestinal Permeability Antigenic Screen
 The Cyrex Labs Array 2 Intestinal Permeability Antigenic Screen is the most accurate intestinal barrier permeability test available and is currently the only lab that measures the immunological response against the intestinal barrier and accepted markers of permeability. The profile includes actin/myosin network IgG, occludin/zonulin IgG, IgA, IgM and lipopolysaccharides (LPS) IgG, IgA, and IgM. Measurements of these antibodies can be used as markers for intestinal permeability. Elevations of occludin/zonulin antibodies indicate breakdown of the intestinal barrier integrity through paracellular (tight junction) pathways. Elevations of actomyosin antibodies indicate breakdown of the intestinal barrier integrity through transcellular (across the intestinal cells) pathways and may also suggest possible autoimmunity against mucosal epithelium and other tissues that contain the actomyosin cytoskeleton. Elevations of LPS antibodies indicate excess penetration of LPS into the bloodstream (leakiness).
The Cyrex Labs Array 2 Intestinal Permeability Antigenic Screen is the most accurate intestinal barrier permeability test available and is currently the only lab that measures the immunological response against the intestinal barrier and accepted markers of permeability. The profile includes actin/myosin network IgG, occludin/zonulin IgG, IgA, IgM and lipopolysaccharides (LPS) IgG, IgA, and IgM. Measurements of these antibodies can be used as markers for intestinal permeability. Elevations of occludin/zonulin antibodies indicate breakdown of the intestinal barrier integrity through paracellular (tight junction) pathways. Elevations of actomyosin antibodies indicate breakdown of the intestinal barrier integrity through transcellular (across the intestinal cells) pathways and may also suggest possible autoimmunity against mucosal epithelium and other tissues that contain the actomyosin cytoskeleton. Elevations of LPS antibodies indicate excess penetration of LPS into the bloodstream (leakiness).
 Conventional Management of Intestinal Permeability
Conventional Management of Intestinal Permeability
There is currently no drug available to treat intestinal permeability and most doctors have not even heard of it. However, this may change in the near future because drug companies are now working on drugs that modulate gut permeability. There is currently a tight junction regulator being developed, intended for the treatment of patients with celiac disease which has just recently passed Phase IIb human drug trial development and will start Phase III clinical trials for the assessment of the oral drug’s efficacy and safety.[287] Once this drug is approved, doctors, or at least gastroenterologists, will become more familiar with intestinal permeability. However, at present, these drugs appear to be limited to approval for the treatment of this one particular intestinal autoimmune disorder (celiac disease). So patients with intestinal permeability with diagnoses of other autoimmune conditions (or any other condition) will not be able to get drug treatments for the foreseeable future.
 Clinical Management of Intestinal Permeability in Functional Medicine
Clinical Management of Intestinal Permeability in Functional Medicine
Fortunately, intestinal permeability usually responds very well to dietary and supplement intervention and can often be well-managed through these interventions alone. The treatment of leaky gut should include an aggressive program designed to break the vicious cycle of leaky gut. This involves a restricted dietary regimen and nutritional supplements that have been shown to help  reduce intestinal inflammation and help repair the intestinal lining. The dietary program may need to be conducted for an extended period of time for more progressed cases, depending on the factors involved in promoting leaky gut. One of the things that will create variables for how long this will take are the mechanisms involved that are causing the leaky gut. If the person is still consuming alcohol or consuming inflammatory foods or has hormonal deficiencies or has blood sugar issues, this will take longer to correct. There will be some people that will have chronic leaky gut for the rest of their life, such as severe autoimmune conditions and people with systemic inflammatory conditions.
reduce intestinal inflammation and help repair the intestinal lining. The dietary program may need to be conducted for an extended period of time for more progressed cases, depending on the factors involved in promoting leaky gut. One of the things that will create variables for how long this will take are the mechanisms involved that are causing the leaky gut. If the person is still consuming alcohol or consuming inflammatory foods or has hormonal deficiencies or has blood sugar issues, this will take longer to correct. There will be some people that will have chronic leaky gut for the rest of their life, such as severe autoimmune conditions and people with systemic inflammatory conditions.
Intestinal Permeability Dietary Restrictions
As discussed above, it is important to avoid foods that tend to aggravate or worsen intestinal permeability when trying to repair the gut barrier and decrease autoimmune responses. Sugars and high glycemic foods can create more damage to the intestinal lining and increase permeability. Gluten (and other grains that cross-react with gluten), dairy and soy can also cause additional inflammation and lead to further damage to the epithelial lining of the GI tract. Lectins (found in grains, legumes, nightshade plants and dairy) have also been shown to damage this epithelial ling. It is important to implement an aggressive and complete dietary program to break the vicious cycle of leaky gut and minimize autoimmune reactions. Here is a list of foods shown to aggravate or worsen intestinal permeability as well as the foods that are generally safely tolerated in individuals with this condition.
- Sugars: including corn syrup, high fructose corn syrup, molasses, honey, chocolate candy, etc.
- High glycemic fruits: watermelon, mango, pineapple, raisins and canned fruit
- Grains: including gluten, wheat, oats, rice, barley, buckwheat, soy, corn, wheat germ, spelt, amaranth, kamut, millet, and quinoa, etc.
- Gluten-containing compounds: including processed salad dressings, ketchup, soy sauce, barbecue sauce, condiments, modified starches, etc.
 Dairy: including milk, whey, eggs, cheeses, creams, and mayonnaise, etc.
Dairy: including milk, whey, eggs, cheeses, creams, and mayonnaise, etc.- Soy: including soy milk, soy sauce, soy protein, etc.
- Alcohol: including beer, wine, sake, cognac, liquors, etc.
- Lectins: including nuts, beans, soy, potatoes, tomato, eggplant, pepper, peanut oil, soy oil, etc.
- Coffee
- Processed foods
- Canned foods
- Most vegetables (except tomato, potatoes and mushrooms): including asparagus, spinach, lettuce, broccoli, beets, cauliflower, carrots, celery artichokes, garlic, onions, zucchini, squash, rhubarb, cucumbers, turnips, watercress, etc.
- Fermented foods: including sauerkraut, kimchi, pickled ginger, mixed pickle, coconut yogurt, kombucha tea, etc.
 Meats: including fish, chicken, beef, lamb, organ meats, etc.
Meats: including fish, chicken, beef, lamb, organ meats, etc.- Low glycemic foods: apricot, plum apple, peach, pear, cherries, berries, etc.
- Coconut: including coconut oil, coconut bitter, coconut milk, etc.
- Herbal teas
- Olives and olive oil
 Nutritional Compounds Shown to Repair the Intestinal Lining
Nutritional Compounds Shown to Repair the Intestinal Lining
There are various plant compounds, vitamins and minerals that have been shown in the literature to have a restorative effect on a damaged intestinal barrier. These include the following: L-glutamine [288-292], deglycyrrhinized licorice [293-303], N-acetyl glucosamine [304-307], aloe leaf extract [308-312], spanish moss [313-317], marshmallow extract [318-321], methylsulfonylmethane (MSM) [322-325], gamma oryzanol [326-330], slippery elm bark [331-334], german chamomile [335-337], marigold flower extract [338-341], glutathione [342-347], zinc carnosine [348], vitamin D [349-351].
 “Regarding intestinal permeability, zinc carnosine caused an approximate threefold increase in gut integrity and repair”[348]
“Regarding intestinal permeability, zinc carnosine caused an approximate threefold increase in gut integrity and repair”[348]
“VDR (vitamin D receptor) plays a critical role in mucosal barrier homeostasis by preserving the integrity of junction complexes and the healing capacity of the colonic epithelium. Therefore, vitamin D deficiency may compromise the mucosal barrier, leading to increased susceptibility to mucosal damage and increased risk of IBD (inflammatory bowel disease).”[349]
 “There is growing appreciation of the importance of the pleiotropic hormone vitamin D in the development of tolerance, immune system defenses, and epithelial barrier integrity” [350]
“There is growing appreciation of the importance of the pleiotropic hormone vitamin D in the development of tolerance, immune system defenses, and epithelial barrier integrity” [350]
“1,25(OH)2D3 (vitamin D) may play a protective role in mucosal barrier homeostasis by maintaining the integrity of junction complexes and in healing capacity of the colon epithelium. 1,25(OH)2D3 may represent an attractive and novel therapeutic agent for the adjuvant therapy of IBD (inflammatory bowel disease).”[351]
References
275. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005 Sep;2(9): 416-22
276. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009 May;1165:195-205.
277. van Ampting MT, Schonewille AJ, Vink C, et al. Intestinal barrier function in response to abundant or depleted mucosal glutathione in Salmonella-infected rats. BMC Physiol. 2009 Apr 17;9:6.
278. Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008 Jul;135(1):194-204.e3.
279. Machowska A, Brzozoweski T, Sliwowski Z, et al. Gastric secretion , proinflammatory cytokines and epidermal growth factor (EGF) in the delayed healing of lingual and gastric ulcerations by testosterone. Inflammopharmacology. 2008 Feb;16(1):40-47.
280. Money SR, Cheron RG, Jaffe BM, Zinner MJ. The effects of thyroid hormones on the formation of stress ulcers in the rat. J Surg Res. 1986 Feb;40(2):176-180.
281. Braniste V, Leveque M, et al. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009 Jul 1;587(Pt 13):3317-3328.
282. Drago F, Montoneri C, Varga C, Laszlo F. Dual effect of female sex steroids on drug-induced gastoduodenal ulcers in the rat. Life Sci. 1999;64(25):2341-2350.
283. Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med. 2008 Jun;8(4):274-281.
284. Zen K, Chen CX, Chen YT, et al. Receptor for advanced glycation endproducts mediates neutrophil migration across intestinal epithelium. J Immunol. 2007 Feb 15;178(4): 2483-2490.
285. Korenaga K, Micci MA, Taglialatela G, Pasricha PJ. Suppression of nNOS expression in rat enteric neurons by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006 May;18(5):392-400.
286. Purohit V, Bode JC, Bode C, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin and medical consequences: summary of a symposium. Alcohol. 2008 Aug;42(5):349-361.
288. Noyer CM, Simon D, Borczuk A, Brandt LJ, Lee MJ, Nehra V. A double-blind placebo-controlled pilot study of glutamine therapy for abnormal intestinal permeability in patients with AIDS. Am J Gastroenterol. 1998 Jun;93(6):972-5.
289. Klimberg VS, Souba WW, Dolson DJ, Salloum RM, Hautamaki RD, Plumley DA, Mendenhall WM, Bova FJ, Khan SR, Hackett RL, et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990 Jul 1;66(1):62-8.
290. Chamorro S, de Blas C, Grant G, Badiola I, Menoyo D, Carabaño R. Effect of dietary supplementation with glutamine and a combination of glutamine-arginine on intestinal health in twenty-five-day-old weaned rabbits. J Anim Sci. 2010 Jan;88(1):170-80.
291. Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann N Y Acad Sci. 2009 May;1165:267-73
292. Kul M, Vurucu S, Demirkaya E, Tunc T, Aydinoz S, Meral C, Kesik V, Alpay F. Enteral glutamine and/or arginine supplementation have favorable effects on oxidative stress parameters in neonatal rat intestine. J Pediatr Gastroenterol Nutr. 2009 Jul;49(1):85-9.
293. Russell RI, Morgan RJ, Nelson LM. Studies on the protective effect of deglycyrrhinised liquorice against aspirin (ASA) and ASA plus bile acidinduced gastric mucosal damage, and ASA absorption in rats. Scand J Gastroenterol Suppl. 1984;92:97-100.
294. Bennett A. Gastric mucosal formation of prostanoids and the effects of drugs. Acta Physiol Hung. 1984;64(3-4):215-7.
295. Morgan RJ, Nelson LM, Russell RI, Docherty C. The protective effect of deglycyrrhi-nized liquorice against aspirin and aspirin plus bile acid-induced gastric mucosal damage, and its influence on aspirin absorption in rats. J Pharm Pharmacol. 1983 Sep;35(9):605-7.
296. Glick L. Deglycyrrhizinated liquorice for peptic ulcer. Lancet. 1982 Oct 9;2(8302):817.
297. van Marle J, Aarsen PN, Lind A, van Weeren-Kramer J. Deglycyrrhizinised liquorice (DGL) and the renewal of rat stomach epithelium. Eur J Pharmacol. 1981 Jun 19;72(2-3):219-25.
298. Datla R, Rao SR, Murthy KJ. Excretion studies of nitrofurantoin and nitrofurantoin with deglycyrrhizinated liquorice. Indian J Physiol Pharmacol. 1981 Jan-Mar;25(1):59-63.
299. Rees WD, Rhodes J, Wright JE, Stamford LF, Bennett A. Effect of deglycyrrhizinated liquorice on gastric mucosal damage by aspirin. Scand J Gastroenterol. 1979;14(5):605-7.
300. Balakrishnan V, Pillai MV, Raveendran PM, Nair CS. Deglycyrrhizinated liquorice in the treatment of chronic duodenal ulcer. J Assoc Physicians India. 1978 Sep;26(9):811-4.
301. D’Imperio N, Giuliani Piccari G, Sarti F, Soffritti M, Spongano P, Benvenuti C, Dal Monte PR. Double-blind trial in duodenal and gastric ulcers. Cimetidine and deglycyrrhizinized liquorice. Acta Gastroenterol Belg. 1978 Jul-Aug;41(7-8):427-34.
302. Bardhan KD, Cumberland DC, Dixon RA, Holdsworth CD. Proceedings: Deglycyrrhizi-nated liquorice in gastric ulcer: a double blind controlled study. Gut. 1976 May;17(5):397.
303. Larkworthy W, Holgate PF. Deglycyrrhizinized liquorice in the treatment of chronic duodenal ulcer. A retrospective endoscopic survey of 32 patients. Practitioner. 1975 Dec;215(1290):787-92.
304. Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010 Apr;56(3):290-9.
305. Gunn-Moore DA, Shenoy CM. Oral glucosamine and the management of feline idiopathic cystitis. J Feline Med Surg. 2004 Aug;6(4):219-25.
306. Seidman EG, Bernotti S, Levy E. Nutritional modulation of gut inflammation. Nestle Nutr Workshop Ser Clin Perform Programme. 2002;7:41-61; discussion 61-5.
307. Sharma R, Schumacher U. Carbohydrate expression in the intestinal mucosa. Adv Anat Embryol Cell Biol. 2001;160:III-IX, 1-91.
308. Chen W, Lu Z, Viljoen A, Hamman J. Intestinal drug transport enhancement by Aloe vera. Planta Med. 2009 May;75(6):587-95.
309. Pogribna M, Freeman JP, Paine D, Boudreau MD. Effect of Aloe vera whole leaf extract on short chain fatty acids production by Bacteroides fragilis, Bifidobacterium infantis and Eubacterium limosum. Lett Appl Microbiol. 2008 May;46(5):575-80.
310. Rosca-Casian O, Parvu M, Vlase L, Tamas M. Antifungal activity of Aloe vera leaves. Fitoterapia. 2007 Apr;78(3):219-22.
311. Iena IaM. [The therapeutic properties of aloe]. Lik Sprava. 1993 Feb-Mar;(2-3):142-5.
312. t’Hart LA, van den Berg AJ, Kuis L, van Dijk H, Labadie RP. An anticomplementary polysaccharide with immunological adjuvant activity from the leaf parenchyma gel of Aloe vera. Planta Med. 1989 Dec; 55(6):509-12.
313. de Queiroga MA, de Andrade LM, Florêncio KC, de Fátima Agra M, da Silva MS, Barbosa-Filho JM, da-Cunha EV. Chemical constituents from Tillandsia recurvata. Fitoterapia. 2004 Jun;75(3-4):423-5.
314. Arslanian RL, Stermitz FR, Castedo L. 3-Methoxy-5-hydroxyflavonols from Tillandsia purpurea. J Nat Prod. 1986 Nov-Dec;49(6):1177-8.
315. Feurt SD, Fox LE. Effects of oral administration of Spanish moss, Tillandsia usneoides L. Science. 1953 Nov 20;118(3073):626-7.
316. Feurt SD, Fox LE. The pharmacological activity of substances extracted from Spanish moss, Tillandsia usneoides L. J Am Pharm Assoc (Baltim). 1952 Aug;41(8):453-4.
317. Webber MG, Lauter WM, Foote PA. A preliminary phytochemical study of Tillandsia usneoides L. (Spanish moss). J Am Pharm Assoc. 1952 May;41(5):230-5.
318. Hage-Sleiman R, Mroueh M, Daher CF. Pharmacological evaluation of aqueous extract of Althaea officinalis flower grown in Lebanon. Pharm Biol. 2011 Mar;49(3):327-33.
319. Deters A, Zippel J, Hellenbrand N, Pappai D, Possemeyer C, Hensel A. Aqueous extracts and polysaccharides from Marshmallow roots (Althea officinalis L.): cellular internalisa-tion and stimulation of cell physiology of human epithelial cells in vitro. J Ethnopharmacol. 2010 Jan 8;127(1):62-9.
320. Kardosová A, Machová E. Antioxidant activity of medicinal plant polysaccharides. Fitoterapia. 2006 Jul;77(5):367-73. Epub 2006 May 24.
321. Wang DF, Shang JY, Yu QH. [Analgesic and anti-inflammatory effects of the flower of Althaea rosea (L.) Cav.]. Zhongguo Zhong Yao Za Zhi. 1989 Jan;14(1):46-8, 64.
322. Iauk L, Lo Bue AM, Milazzo I, Rapisarda A, Blandino G. Antibacterial activity of medicinal plant extracts against periodontopathic bacteria. Phytother Res. 2003 Jun;17(6):599-604.
323. Satia JA, Littman A, Slatore CG, Galanko JA, White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol Biomarkers Prev. 2009 May;18(5):1419-28.
324. Brien S, Prescott P, Bashir N, Lewith H, Lewith G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthritis Cartilage. 2008 Nov;16(11):1277-88.
325. Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002 Feb;7(1):22-44.
326. Jariwalla RJ. Rice-bran products: phytonutrients with potential applications in preventive and clinical medicine. Drugs Exp Clin Res. 2001;27(1):17-26.
327. Sugano M, Tsuji E. Rice bran oil and human health. Biomed Environ Sci. 1996 Sep;9(2-3):242-6.
328. Hirose M, Ozaki K, Takaba K, Fukushima S, Shirai T, Ito N. Modifying effects of the naturally occurring antioxidants gamma-oryzanol, phytic acid, tannic acid and n-tritriacontane-16, 18-dione in a rat wide-spectrum organ carcinogenesis model. Carcinogenesis. 1991 Oct;12(10):1917-21.
329. Wheeler KB, Garleb KA. Gamma oryzanol-plant sterol supplementation: metabolic, endocrine, and physiologic effects. Int J Sport Nutr. 1991 Jun;1(2):170-7.
330. Itaya K, Kitonaga J, Ishikawa M. [Studies of gamma-oryzanol. The antiulcerogenic action]. Nippon Yakurigaku Zasshi. 1976 Nov;72(8):1001-11.
331. Brown AC, Hairfield M, Richards DG, McMillin DL, Mein EA, Nelson CD. Medical nutrition therapy as a potential complementary treatment for psoriasis-five case reports. Altern Med Rev. 2004 Sep;9(3):297-307.
332. Choi HR, Choi JS, Han YN, Bae SJ, Chung HY. Peroxynitrite scavenging activity of herb extracts. Phytother Res. 2002 Jun;16(4):364-7.
333. Majchrowicz MA. Essiac. Notes Undergr. 1995 Winter;(no 29):6-7.
334. Gill RE, Hirst EL, Jones JK. Constitution of the mucilage from the bark of Ulmus fulva (slippery elm mucilage); the sugars formed in the hydrolysis of the methylated mucilage. J Chem Soc. 1946 Nov:1025-9.
335. Bezerra SB, Leal LK, Pinto NA, Campos AR. Bisabolol-induced gastroprotection against acute gastric lesions: role of prostaglandins, nitric oxide, and KATP+ channels. J Med Food. 2009 Dec;12(6):1403-6.
336. Moura Rocha NF, Venâncio ET, Moura BA, et al. Gastroprotection of (-)-alphabisa-bolol on acute gastric mucosal lesions in mice: the possible involved pharmacological mechanisms. Fundam Clin Pharmacol. 2009 Aug 3.
337. Pereira RP, Fachinetto R, de Souza Prestes A, et al. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem Res. 2009 May;34(5):973-83.
338. Madisch A, Holtmann G, Mayr G, Vinson B, Hotz J. Treatment of functional dyspepsia with a herbal preparation. A double-blind, randomized, placebo-controlled, multicenter trial. Digestion. 2004;69(1):45-52.
339. Elango G, Rahuman AA, Kamaraj C, Zahir AA, Bagavan A. Studies on effects of indigenous plant extracts on filarial vector Culex tritaeniorhynchus Giles. Parasitol Res. 2010 Apr 7.
340. Bashir S, Gilani AH. Studies on the antioxidant and analgesic activities of Aztec marigold (Tagetes erecta) flowers. Phytother Res. 2008 Dec;22(12):1692-4.
341. Wang M, Tsao R, Zhang S, Dong Z, Yang R, Gong J, Pei Y. Antioxidant activity, mutagenicity/ anti-mutagenicity, and clastogenicity/anticlastogenicity of lutein from marigold flowers. Food Chem Toxicol. 2006 Sep;44(9):1522-9.
342. Martín-Venegas R, Brufau MT, Guerrero-Zamora AM, Mercier Y, Geraert PA, Ferrer R. The methionine precursor DL-2-hydroxy-(4-methylthio) butanoic acid protects intestinal epithelial barrier function. Food Chem. 2013 Dec 1;141(3):1702-9.
343. Flamant M, Aubert P, Rolli-Derkinderen M, et al. Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione. Gut. 2011 Apr;60(4):473-84.
344. van Ampting MT, Schonewille AJ, Vink C, Brummer RJ, van der Meer R, Bovee-Oudenhoven IM. Intestinal barrier function in response to abundant or depleted mucosal glutathione in Salmonella-infected rats. BMC Physiol. 2009 Apr 17:9:6
345. Maeda T, Miyazono Y, Ito K, Hamada K, Sekine S, Horie T. Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate treated rats. Cancer Chemother Pharmacol. 2010 May;65(6):1117-2
346. Savidge TC, Newman P, Pothoulakis C, Ruhl A, Neunlist M, Bourreille A, Hurst R, Sofroniew MV. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007 Apr;132(4):1344-1358
347. The role of the glutathione antioxidant system in gut barrier failure in a rodent model of experimental necrotizing enterocolitis. Surgery. 2004 Sep;136(3):557-66
348. Mahmood A, FitzGerald AJ, Marchbank T, Ntatsaki E, Murray D, Ghosh S, Playford RJ. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. 2007 Feb;56(2):168-75. Epub 2006 Jun 15.
349. Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastroint. Liver Physiol. 2008 Jan; 294(1):G208-16
350. Vassallo MF, Camargo CA Jr. Potential mechanisms for the hypothesized link between sunshine, vitamin D, and food allergy in children. J Allergy Clin Immunol. 2010 Aug;126 (2):217-22
351. Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, Li C, Shih DQ, Zhang X. Protective role of 1,25(OH)2 vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterol. 2012 May 30;12:57.