 Recent research has discovered some fascinating connections between the gut and several other organs of the body. In this segment, we review some of these connections between the gut and the liver, immune system, brain and thyroid that have been recently identified in the literature. For a review of the basic functions of the gastrointestinal system, click here: Functions of the Gastrointestinal System.
Recent research has discovered some fascinating connections between the gut and several other organs of the body. In this segment, we review some of these connections between the gut and the liver, immune system, brain and thyroid that have been recently identified in the literature. For a review of the basic functions of the gastrointestinal system, click here: Functions of the Gastrointestinal System.
Introduction
One of the things we learn in our medical training is that the body is composed of separate organs and organ systems that operate independently and are not closely related and this often makes us inefficient in managing conditions related to these organ systems. We all learn neurology, immunology, and gastroenterology as separate fields of study that can be studied independently but they really aren’t separate systems that can be fully appreciated if they are studied in isolation. The organ systems of the body are all interconnected and inter-influential. In our current healthcare system, we have various specialists that are highly specialized in their field but for the most part, they don’t communicate with other specialists outside of their field, even when it comes to mutual patients. No one else is allowed to talk about their specialty because they feel they are the specialist in that field. However, the body doesn’t work like that. All the organs of the body communicate and influence other organs and the gut is no exception. In fact, the gut communicates with possibly every other organ in the body.
 Gut Connections with Other Organs
Gut Connections with Other Organs
In this segment, we review some of the connections the recent research is showing between the gut and the following organs of the body:
- Liver
- Immune system
- Brain
- Thyroid
 Gut-Liver Connection
Gut-Liver Connection
There are several relationships between the GI tract and the liver. It is common knowledge when toxins and xenobiotics are absorbed into the body via food or water, they enter the hepatic portal vein which transports these compounds to the liver before distribution to the body. The liver is the primary organ for detoxification of toxicants in our food and environment and for the majority of detoxification enzymes in the body. The primary mechanism in the body for removing lipid-soluble toxins is the hepatic biotransformation (liver detoxification) system which functions to convert lipid-soluble substances to water-soluble substances so they can be excreted via the urine.
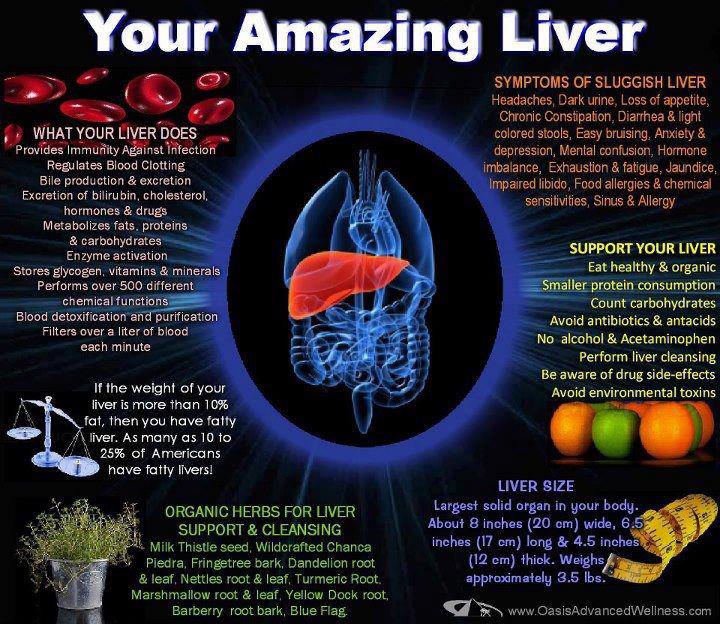 Abnormal absorption of nutrients in the small intestine can affect liver function. For example, increased gut permeability leads to increased toxic load on the liver. When the intestinal barrier function becomes disrupted, toxins and undigested food compounds enter the circulation via the hepatic portal vein and lead to increased demands on the liver. This can lead to compromised liver function in its ability to transform toxins and increased toxic load in the body and result in a wide range of symptomology. Clinicians see problems secondary to liver detoxification on a daily basis, whether they recognize them or not. It is a very common problem in modern society where most of our food is contaminated with antibiotics, hormones, pesticides and herbicides. Additionally, malabsorption of nutrients can also affect liver function. The liver relies on many nutrients to perform its various functions, such as B vitamins, antioxidants, cofactors and coenzymes and if these are not being absorbed in the gut, liver function can be compromised.
Abnormal absorption of nutrients in the small intestine can affect liver function. For example, increased gut permeability leads to increased toxic load on the liver. When the intestinal barrier function becomes disrupted, toxins and undigested food compounds enter the circulation via the hepatic portal vein and lead to increased demands on the liver. This can lead to compromised liver function in its ability to transform toxins and increased toxic load in the body and result in a wide range of symptomology. Clinicians see problems secondary to liver detoxification on a daily basis, whether they recognize them or not. It is a very common problem in modern society where most of our food is contaminated with antibiotics, hormones, pesticides and herbicides. Additionally, malabsorption of nutrients can also affect liver function. The liver relies on many nutrients to perform its various functions, such as B vitamins, antioxidants, cofactors and coenzymes and if these are not being absorbed in the gut, liver function can be compromised.
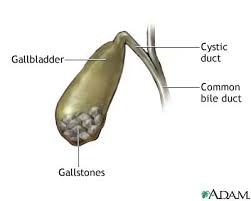 In addition to regulating many complex biochemical reactions involved in carbohydrate, protein and lipid metabolism, the liver synthesizes bile which is important in lipid digestion and absorption. The gall bladder stores this bile which is ejected into the biliary tract and enters the small intestine. As described in part 8a, bile is used to emulsify lipids which allows for absorption into the body in the small intestine. Problems with the production of healthy bile leads to problems with fat digestion and risk of gallstones or biliary obstruction, which can lead to acute pancreatitis. Bile is also important in the elimination of toxins from the body. The liver deposits metabolized hormones, drugs and xenobiotics into the bile which enters the duodenum of the small intestine for elimination from the body through the GI tract. Problems with biliary stasis, unhealthy bile production or bowel transit time can all lead to problems with elimination of toxins from the body.
In addition to regulating many complex biochemical reactions involved in carbohydrate, protein and lipid metabolism, the liver synthesizes bile which is important in lipid digestion and absorption. The gall bladder stores this bile which is ejected into the biliary tract and enters the small intestine. As described in part 8a, bile is used to emulsify lipids which allows for absorption into the body in the small intestine. Problems with the production of healthy bile leads to problems with fat digestion and risk of gallstones or biliary obstruction, which can lead to acute pancreatitis. Bile is also important in the elimination of toxins from the body. The liver deposits metabolized hormones, drugs and xenobiotics into the bile which enters the duodenum of the small intestine for elimination from the body through the GI tract. Problems with biliary stasis, unhealthy bile production or bowel transit time can all lead to problems with elimination of toxins from the body.
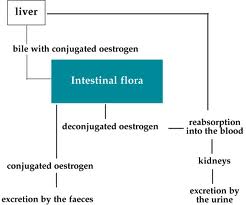 In addition, gut dysbiosis (imbalance of the gut bacteria) can affect the liver’s detoxification capacity. Dysbiosis can increase activity of beta-glucuronidase, an enzyme of the gut, which cleaves off the conjugated portion of the toxins, such as estrogens, that have already been conjugated (detoxified) by the liver. This means the liver’s work in eliminating toxic hormones from the body, such as estrogen and other xenoestrogens, is reversed, and this places increased demands on the liver to perform its job of conjugation of hormones all over again.[63] Glucuronidases liberate toxins and mutagens that have been conjugated (glucuronidated) in the liver and excreted into the gut with the bile. This can lead to high local concentrations of carcinogenic compounds within the gut, thus increasing the risk of carcinogenesis.[63]
In addition, gut dysbiosis (imbalance of the gut bacteria) can affect the liver’s detoxification capacity. Dysbiosis can increase activity of beta-glucuronidase, an enzyme of the gut, which cleaves off the conjugated portion of the toxins, such as estrogens, that have already been conjugated (detoxified) by the liver. This means the liver’s work in eliminating toxic hormones from the body, such as estrogen and other xenoestrogens, is reversed, and this places increased demands on the liver to perform its job of conjugation of hormones all over again.[63] Glucuronidases liberate toxins and mutagens that have been conjugated (glucuronidated) in the liver and excreted into the gut with the bile. This can lead to high local concentrations of carcinogenic compounds within the gut, thus increasing the risk of carcinogenesis.[63]
 Gut-Immune Connection
Gut-Immune Connection
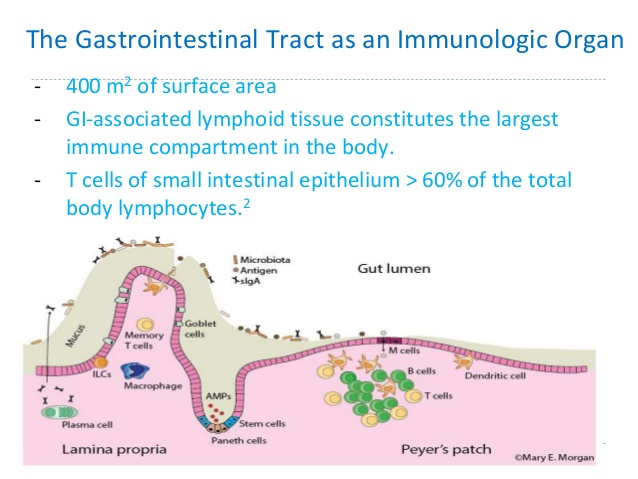
As described above, the gut-associated lymphoid tissue is an important part of our immune system which is located in the wall of the gut and protects us from a wide variety of pathogens and potentially harmful compounds. The primary purpose of the GALT is to provide the first line of defense against foreign invaders such as food antigens (which elicit immune responses) and pathogenic bacteria and parasites. The GALT has two layers of defense to a foreign pathogen or antigen: localized secretory IgA (sIgA) responses and the systemic IgE and IgG responses. Together, these two responses account for the majority of the body’s immunoglobulins (Ig) which are important proteins needed for immune responses.
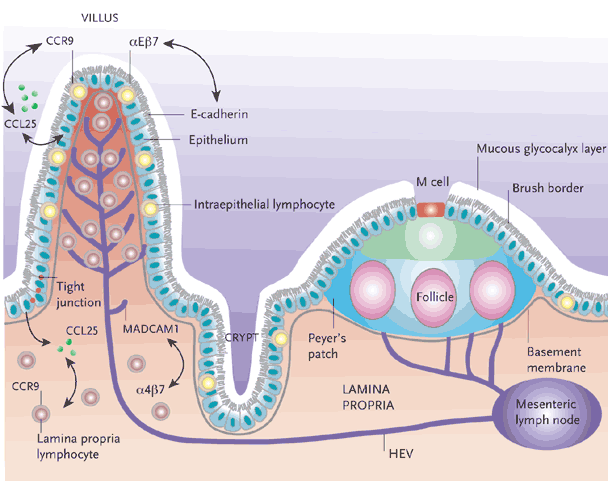 The GALT serves as a containment system, preventing potentially harmful antigens from reaching systemic circulation, and induces systemic tolerance against intestinal antigens by a process that involves IgA secretion and the induction of regulatory T-cells. As the predominant immunoglobulin on the surface of the GI mucosa, sIgA cells can effectively prevent infection, neutralize viruses and remove antigens before they can cross the mucosal barrier and reach circulation. Antigens and foreign invaders which escape the sIgA surveillance can enter the mucosal layer where the GALT provides the second layer of defense. The interaction of antigen-specific IgE and IgG induces a systemic immune response in which antibodies are generated, cytokines are produced and the full immune response is engaged.
The GALT serves as a containment system, preventing potentially harmful antigens from reaching systemic circulation, and induces systemic tolerance against intestinal antigens by a process that involves IgA secretion and the induction of regulatory T-cells. As the predominant immunoglobulin on the surface of the GI mucosa, sIgA cells can effectively prevent infection, neutralize viruses and remove antigens before they can cross the mucosal barrier and reach circulation. Antigens and foreign invaders which escape the sIgA surveillance can enter the mucosal layer where the GALT provides the second layer of defense. The interaction of antigen-specific IgE and IgG induces a systemic immune response in which antibodies are generated, cytokines are produced and the full immune response is engaged.
 The GALT is also instrumental in the development of immune or oral tolerance to harmless antigens in the diet and commensal gut bacteria. Oral tolerance is an important process in which the body becomes able to recognize food molecules and not respond to them with a full-fledged antibody-immune response. Oral tolerance is also stimulated by the commensal gut bacteria.
The GALT is also instrumental in the development of immune or oral tolerance to harmless antigens in the diet and commensal gut bacteria. Oral tolerance is an important process in which the body becomes able to recognize food molecules and not respond to them with a full-fledged antibody-immune response. Oral tolerance is also stimulated by the commensal gut bacteria.
Gut permeability has a direct impact on systemic immune responses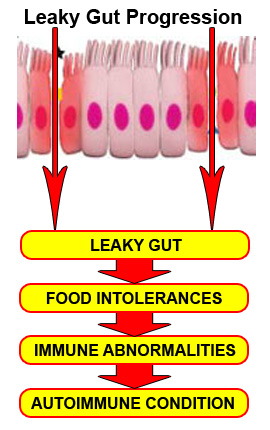 and chronic inflammation. Increased gut permeability (leaky gut) leads to increased antigens penetrating the gut barrier and entering circulation. When there is antigen penetration, there is antibody response to these antigens, causing immune activation and various inflammatory responses. There are many toxic compounds which can enter circulation due to a breached gut barrier which have been identified, such as lipopolysaccharides (LPS). LPS are end-products of intestinal bacteria metabolism that have been well-studied and widely recognized to produce toxic inflammatory effects when they enter circulation.[64-66] If this chronic inflammation continues unabated, the individual becomes at risk for immune dysregulation and autoimmunity.[67-68] The chronic intestinal inflammation leads to further intestinal permeability which becomes a vicious cycle.
and chronic inflammation. Increased gut permeability (leaky gut) leads to increased antigens penetrating the gut barrier and entering circulation. When there is antigen penetration, there is antibody response to these antigens, causing immune activation and various inflammatory responses. There are many toxic compounds which can enter circulation due to a breached gut barrier which have been identified, such as lipopolysaccharides (LPS). LPS are end-products of intestinal bacteria metabolism that have been well-studied and widely recognized to produce toxic inflammatory effects when they enter circulation.[64-66] If this chronic inflammation continues unabated, the individual becomes at risk for immune dysregulation and autoimmunity.[67-68] The chronic intestinal inflammation leads to further intestinal permeability which becomes a vicious cycle.
 Gut-Brain Connection
Gut-Brain Connection
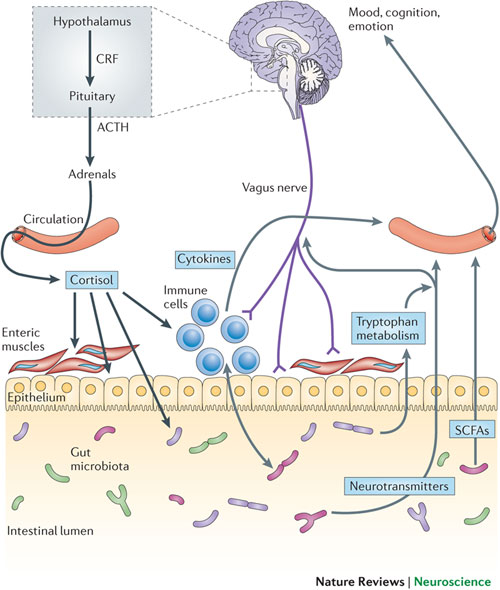
Have you ever felt so emotional that you felt something in your stomach? Some people will see something or think of something that makes them so sick that they throw up. Have you ever been on a roller coaster that makes you sick and want to throw up? This is due to regions in the brain firing into the enteric nervous system of the gut. Have you ever experienced eating too late at night or overeating and having nightmares? This is due to stress receptors in the gut firing into the amygdala, the fear centers of the brain that cause nightmares. Have you ever eaten something and your mood changes? These are opioids, cytokines and chemicals being released into the brain. Why is it that almost every child with a learning disorder has poor digestion and a bad gut? All these are examples of the brain-gut connection. The recent literature is showing a strong connection between the brain and the gut which is now being referred to as the “brain-gut axis”.
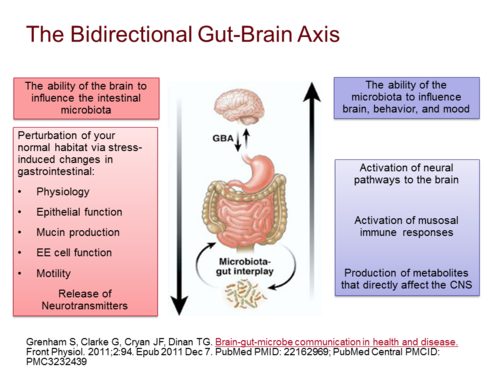 “These results show that this form of early life stress results in an altered brain-gut axis and is, therefore, an important model for investigating potential mechanistic insights into stress-related disorders, including depression and IBS”[69]
“These results show that this form of early life stress results in an altered brain-gut axis and is, therefore, an important model for investigating potential mechanistic insights into stress-related disorders, including depression and IBS”[69]
It is now known that a large percentage of patients with abnormal bowel function often suffer from stress-related disorders or other brain disorders.
“50%-90% of IBS patients that seek treatment have psychiatric disorders, including panic disorder, generalized anxiety disorder, social phobia, post-traumatic stress disorder, and major depression.”[70]
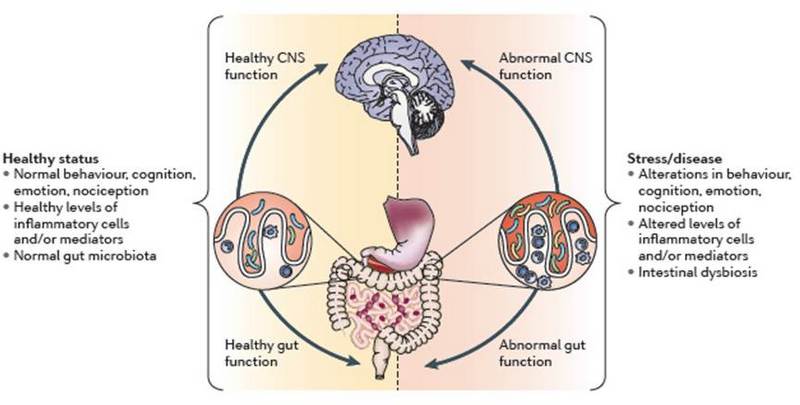 Poor Brain Function Leads To Poor Gut Function
Poor Brain Function Leads To Poor Gut Function
One of the earliest signs of poor brain health is not cognitive, but decreased digestive function. About 90% of the brain’s output enters into a region of the brain called the pontomedullary region located in the lower brainstem and this fires into the vagus nerve which largely controls digestive function. The vagus allows for gut motility, enzyme secretion, and normal bowel activity. When a person loses the ability to fire into the brainstem, they will have decreased vagal output and decreased gut function. If you can’t move your food, you end up with dysbiosis (imbalance of beneficial gut flora), yeast overgrowths, enzyme deficiencies, and inability to contract the gallbladder. This will manifest as abdominal pain or discomfort, constipation, loose stools or diarrhea, gas, bloating, food sensitivities and difficulty digesting meals.
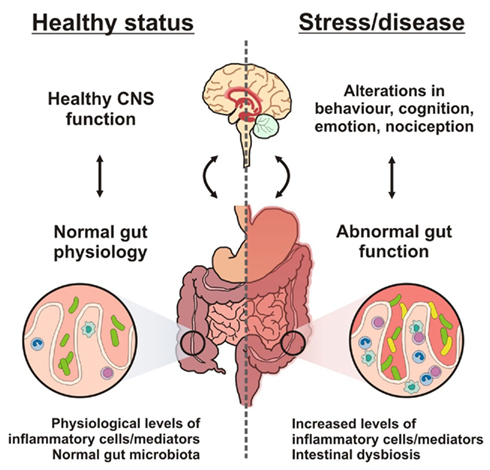 “Brain alteration of neurotransmitters leads to abnormal gastric enzyme secretion. Brain neurochemistry is important for regulating enzyme secretion in the brain-gut relationship.”[71]
“Brain alteration of neurotransmitters leads to abnormal gastric enzyme secretion. Brain neurochemistry is important for regulating enzyme secretion in the brain-gut relationship.”[71]
Doctors will often diagnose conditions of abdominal pain and irregular bowel movements, including constipation or diarrhea, as “irritable bowel syndrome” or IBS. We are now discovering that IBS involves dysfunction of the brain and the gut’s enteric nervous system (also referred to as “the second brain”).
“IBS is one of the most common functional gastrointestinal disorders worldwide is thought to be the result of disturbed neural function along the brain-gut axis” [72]
When a person loses the integrity of their brain-gut axis, it is common to see symptoms of constipation and poor gut motility. They have to use laxatives and drink coffee to have bowel movements. People that lose bowel function tend to drink coffee all the time because coffee produces a cholinergic effect and increases gut motility and blood flow to brain. They are basically trying to rev up their brain-gut axis. But one of the things people with poor brain function have besides constipation and enzyme deficiency is leaky gut.
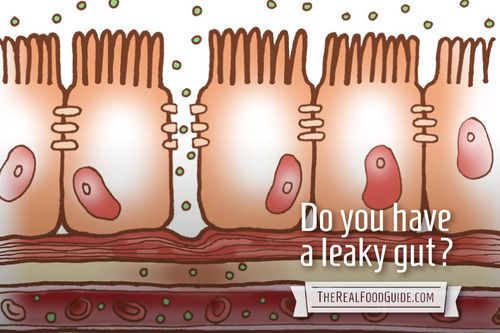 One of the things poor brain function causes is intestinal permeability or “leaky gut”. This is loss of the barrier function of the gut that protects our bodies from large molecules that should normally not enter circulation, such as undigested food proteins, bacteria and other toxins. This can lead to gastritis or intestinal ulcers. When the integrity of the gut barrier becomes compromised, undigested food particles can enter circulation causing immune responses and leading to multiple food sensitivities.[73]
One of the things poor brain function causes is intestinal permeability or “leaky gut”. This is loss of the barrier function of the gut that protects our bodies from large molecules that should normally not enter circulation, such as undigested food proteins, bacteria and other toxins. This can lead to gastritis or intestinal ulcers. When the integrity of the gut barrier becomes compromised, undigested food particles can enter circulation causing immune responses and leading to multiple food sensitivities.[73]
“These results indicate that the brain is important for regulating development of intestinal ulcers, and that classical neurotransmitters Ach, NE and 5-HT are involved”[74]
 Poor Gut Function Leads To Poor Brain Function
Poor Gut Function Leads To Poor Brain Function
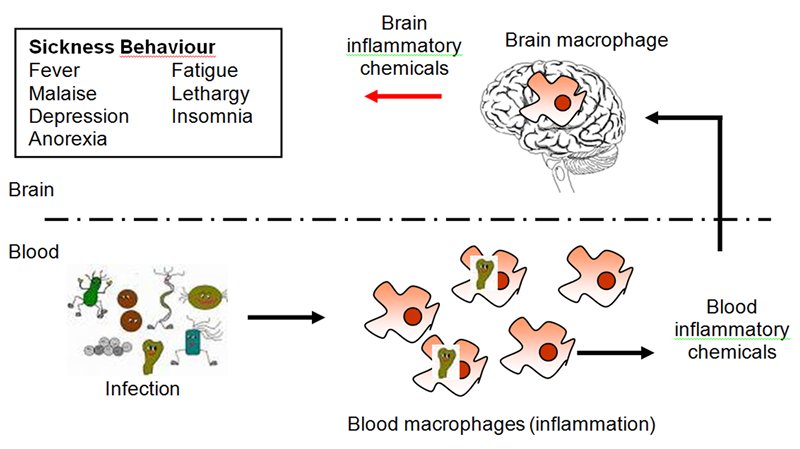
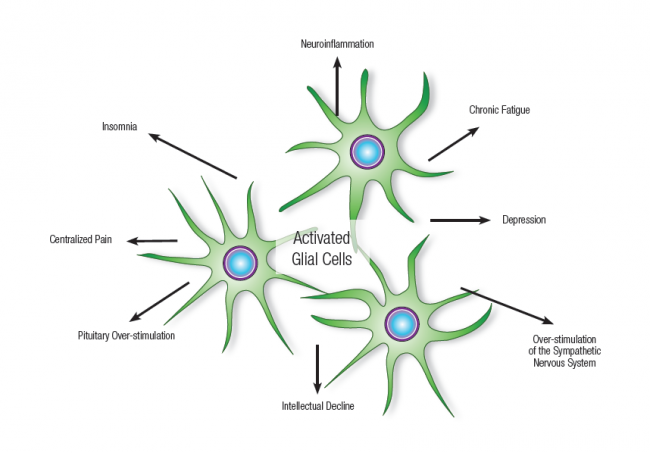
The combination of intestinal permeability and undigested proteins and toxins entering circulation causes systemic inflammation and releases cytokines which have been shown to cross the blood brain barrier and activate the microglial cells of the brain.[75] The microglial cells, when they get activated, create surrounding neuroinflammation in the brain. The neuroinflammation causes the surrounding neurons to lose their nerve conductance so they don’t fire as fast. The brain has no pain fibers so the chief indication of neuroinflammation is slow brain function or “brain fog”. This is when someone tries to get to a thought but they can’t get to it. They have difficulty thinking clearly and have problems with memory and recall.
 “When cytokine signals from the peripheral immune system reach the CNS, microglia become activated and propagate the inflammatory response throughout the brain.”[76]
“When cytokine signals from the peripheral immune system reach the CNS, microglia become activated and propagate the inflammatory response throughout the brain.”[76]
If this glial activation is sustained for a long period of time, it will eventually degenerate the brain and the enteric nervous system. When someone has a gut on fire and brain on fire, they will have bloating and food sensitivities with brain fog or poor brain function. Their brain function is affected by food they can’t digest. This leads to a vicious cycle of brain degeneration, leaky gut, increased cytokine activation and further brain degeneration.
Once you get this inflammatory response in the gut, there is a consequence of inflammation in the brain. There is now evidence that major depression disorder (MDD) is accompanied by an activation of the inflammatory response system in the brain and that pro-inflammatory cytokines and lipopolysaccharide (LPS) from the gut may induce depressive symptoms.
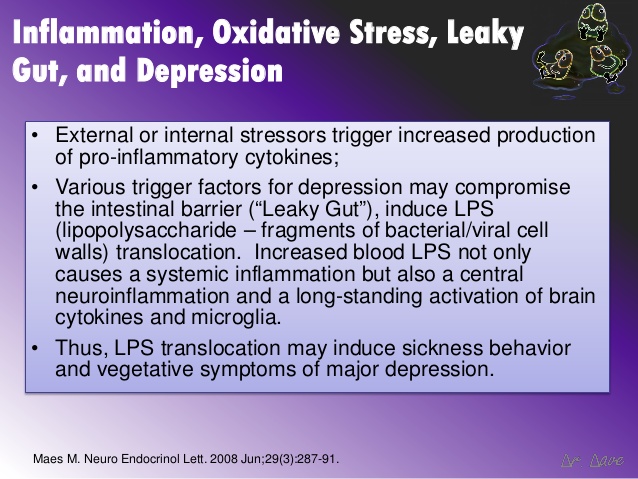 “The results showed that intestinal mucosa dysfunction characterized by an increase translocation of enteric bacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression.”[77]
“The results showed that intestinal mucosa dysfunction characterized by an increase translocation of enteric bacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression.”[77]
A Mayo Clinic study found the observed prevalence of multiple sclerosis (an autoimmune disease that destroys the central nervous system) at the onset of inflammatory bowel disease was 3.7 times higher than expected.[78] A study published in the Lancet demonstrated that the frequency of focal white-matter lesions in patients with inflammatory bowel disease is almost as high as that in patients with multiple sclerosis.[79] Another study essentially concluded that there is no way to have neurodegeneration in the brain and not have neurodegeneration in the gut.[80]
 Brain-Gut Peptides
Brain-Gut Peptides
During the past few years, several brain-gut peptides have emerged as important mediators of communication in the brain-gut axis. Peptides including substance P, neurotensin and galanin are produced in the brain and enteric nervous system are involved in regulating intestinal infection [81]. Cholesystokinin (CCK) is a brain-gut peptide with broad biological activities and serves as a regulating hormone in the digestive system. It also plays a central role in the central nervous system as a neurotransmitter or a neuromodulator. Recently many reports have demonstrated CCK genetic variations with psychiatric states such as schizophrenia, depressive disorder, suicidal behavior and Parkinson’s disease.[82]
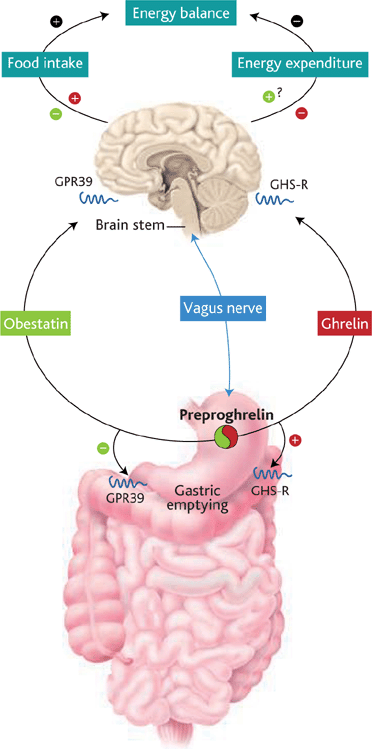
The gut hormone and neuropeptide ghrelin affects energy balance and growth hormone release through hypothalamic action in the brain.[83] One paper reported that ghrelin enters the hippocampus and binds to neurons of the hippocampal formation, where it promotes dendritic synapse formation and generation of long-term potentiation to enhance learning and memory processes![83] Neurotensin (NT), is an endogenous brain-gut peptide which has a close functional relationship with the dopamine system. Emerging research demonstrates it may play a role in psychiatric and neurological disease.[84]
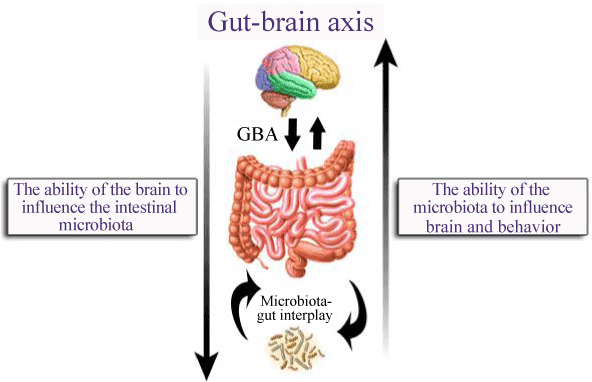 Brain Influence on Gut Flora
Brain Influence on Gut Flora
“The brain can influence commensal organisms (enteric microbiota) indirectly via changes in gastrointestinal motility and secretion, and intestinal permeability, or directly, via signaling molecules released into the gut lumen from cells of the lamina propria.
“Disruption of the bi-directional interactions between the enteric microbiota and the brain may be involved in the pathophysiology of acute and chronic gastrointestinal disease states, including functional and inflammatory bowel disorders.”[85]
 Gut Flora Influence on Brain
Gut Flora Influence on Brain
“While bi-directional brain-gut interactions are well known mechanisms for the regulation of gut function in both healthy and diseased states, a role of the enteric flora—including both commensal and pathogenic organisms—in these interactions has only been recognized in the past few years.”[85]
“The absence of probiotic bacteria in the gut can have adverse effects not only locally in the gut, but also has been shown to affect central brain hypothalamic-pituitary-adrenal and monoaminergic activity, features that have been implicated in the etiology of depression.
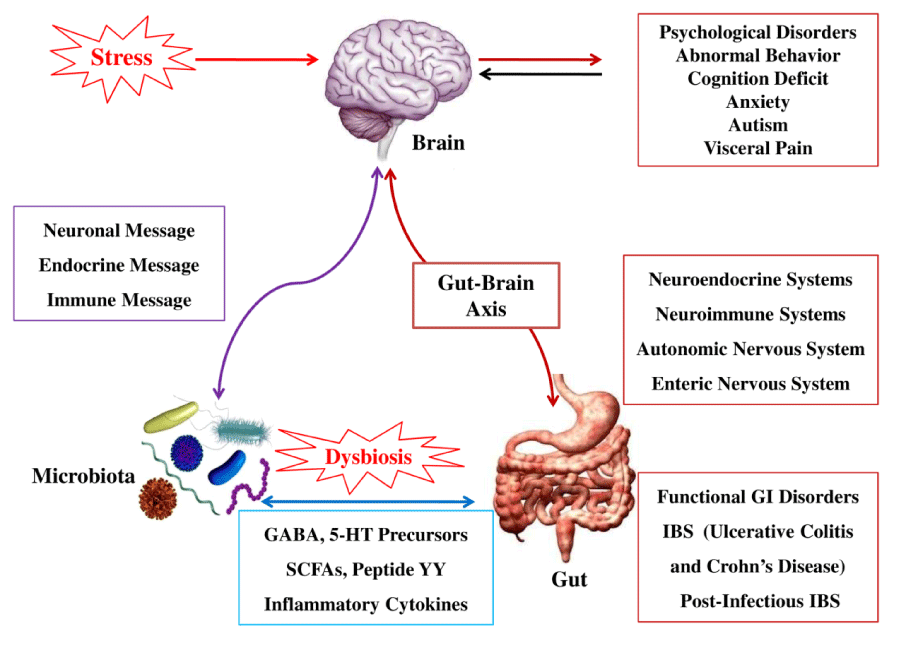 “The attenuation of pro-inflammatory immune responses and the elevation of the serotonergic precursor tryptophan by bifidobacteria (probiotic) treatment, provides encouraging evidence in support of the proposition that this probiotic may possess anti-depressant properties.”[86]
“The attenuation of pro-inflammatory immune responses and the elevation of the serotonergic precursor tryptophan by bifidobacteria (probiotic) treatment, provides encouraging evidence in support of the proposition that this probiotic may possess anti-depressant properties.”[86]
This paper shows two important discoveries. First, bifidobacteria (an important commensal gut bacteria) actually impacts the serotonergic system. In other words, it impacts your ability to make serotonin, the primary neurotransmitter associated with depression. Secondly, imbalances in gut microflora alone can disrupt the gut-brain axis sufficiently to cause depression. These recent discoveries of the gut-brain axis are making it increasingly clear how interdependent the function of the gut and brain really are.
 Gut-Thyroid Connection
Gut-Thyroid Connection
“Thyroid hormones have an effect on the gastrointestinal tract at all levels of organization and clinicians have long recognized the associations that exist between gastrointestinal symptoms and thyroid disease.”[87]
Thyroid Influences on the Gut
Thyroid hormone has a direct impact on gut motility, gut transit time and risk of various gut disorders, including risk of gallstones. The most common gut-related complaints reported in thyroid diseases are those due to disordered bowel function, in particular, alteration in motility in the gastrointestinal tract and bowel regularity. The motor activity of the gastrointestinal tract is increased in hyperthyroidism and decreased in hypothyroidism. The sluggish motility in hypothyroidism may directly lead to constipation and even intestinal obstruction. Diarrhea and steatorrhea (fat in stool) commonly occur in hyperthyroidism.[87,88]
 The level of gastric acid secretion has also been reported to vary greatly with both hypo- and hyperthyroid states. Hypo- and hyperthyroidism are frequently associated with gastritis (inflammation of the stomach lining) and hypochlorhydria (diminished acid output).[87,88] This often results in acid reflux or GERD. Gastric emptying and esophageal activity are also prolonged in hypothyroidism.[89] Patients with hypothyroidism may also suffer from rectal prolapse, chronic constipation, colonic dilatation (enlargement), and fecal impaction. Rapid gastric emptying and rapid transit time have been associated with hyperthyroidism. [90]
The level of gastric acid secretion has also been reported to vary greatly with both hypo- and hyperthyroid states. Hypo- and hyperthyroidism are frequently associated with gastritis (inflammation of the stomach lining) and hypochlorhydria (diminished acid output).[87,88] This often results in acid reflux or GERD. Gastric emptying and esophageal activity are also prolonged in hypothyroidism.[89] Patients with hypothyroidism may also suffer from rectal prolapse, chronic constipation, colonic dilatation (enlargement), and fecal impaction. Rapid gastric emptying and rapid transit time have been associated with hyperthyroidism. [90]
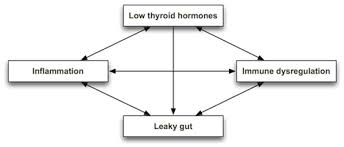 Hypothyroidism is also a major risk factor for intestinal permeability or leaky gut syndrome.
Hypothyroidism is also a major risk factor for intestinal permeability or leaky gut syndrome.
“Lack of thyroid hormone stimulation of gastric and intestinal cells leads to ulcerations and intestinal permeability known as “leaky gut syndrome”. Additionally, endoscopic examination of gastric ulcers found low T3, low T4, and abnormal levels of reverse T3 on gastric ulcerative tissues.”[90a]
“Both thyroxine (T4) and triiodothyronine (T3) have been shown to protect the intestinal mucosal lining from stress-induced intestinal lining injury.”[90b]
 For decades, one well-known risk factor for the development of gallstones has been hypothyroidism. Recent studies have reported that the risk in particular for stone formation in the common bile duct (CBD) increases substantially in clinical (and subclinical) hypothyroidism.[91] There are multiple factors that may contribute to the formation and/or accumulation of CBD stones in hypothyroid patients, including altered bile composition, diminished bile secretion, and emptying of the biliary tract.[91]
For decades, one well-known risk factor for the development of gallstones has been hypothyroidism. Recent studies have reported that the risk in particular for stone formation in the common bile duct (CBD) increases substantially in clinical (and subclinical) hypothyroidism.[91] There are multiple factors that may contribute to the formation and/or accumulation of CBD stones in hypothyroid patients, including altered bile composition, diminished bile secretion, and emptying of the biliary tract.[91]
 Gut Influences on the Thyroid
Gut Influences on the Thyroid
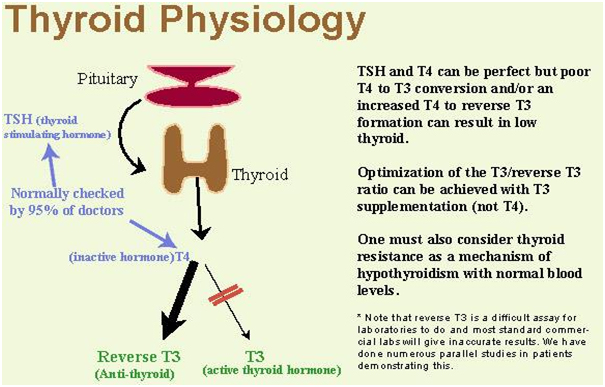
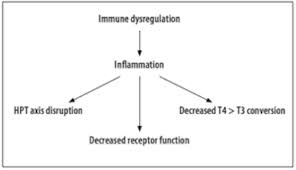
Thyroid hormones are produced as primarily T4 in the thyroid and have to be converted from T4 to T3 hormone since T3 is the primary active thyroid hormone in the body. Most thyroid responses are due to the binding of T3 to thyroid receptors on the various cells of the body, not T4. 93% of what the thyroid gland produces is T4 while only 7% is T3. This means that the majority of thyroid hormone produced has to be converted to an active form in order to have a thyroid response. The enzyme that converts T4 to T3 is 5’deiodinase which is found in every major tissue, especially the liver. A defect in this enzyme activity causes an underconversion problem. Gut infections or chronic inflammation in the gut can cause elevations in cytokine activity which can negatively impact conversion of T4 to T3 causing low T3 hormone levels and a functional hypothyroid condition.
 Another way that gut activity influences thyroid hormone levels is through gut enzyme activity acting on T4 hormone. As stated, T4 gets converted to T3 primarily in the liver. But only 60% of T4 gets converted to actual T3 in normal healthy metabolism. About 20% of T4 gets converted to reverse T3 (rT3). RT3 is inactive thyroid hormone and has no potential to become active thyroid hormone (T3) ever again. Certain conditions can increase the amount of rT3 produced such as intestinal permeability or inflammation in the gut, resulting in less potential T3. The remaining 20% of T4 gets converted to T3 sulfate (T3S) or T3 acetic acid (T3AC). But unlike rT3, these inactive forms of thyroid hormone can become active again. This occurs by interacting with normal gut bacteria. Gut bacteria have the ability to convert T3S and T3AC back into active T3! Therefore, normal thyroid hormone levels rely on a healthy gut environment and gut metabolism.
Another way that gut activity influences thyroid hormone levels is through gut enzyme activity acting on T4 hormone. As stated, T4 gets converted to T3 primarily in the liver. But only 60% of T4 gets converted to actual T3 in normal healthy metabolism. About 20% of T4 gets converted to reverse T3 (rT3). RT3 is inactive thyroid hormone and has no potential to become active thyroid hormone (T3) ever again. Certain conditions can increase the amount of rT3 produced such as intestinal permeability or inflammation in the gut, resulting in less potential T3. The remaining 20% of T4 gets converted to T3 sulfate (T3S) or T3 acetic acid (T3AC). But unlike rT3, these inactive forms of thyroid hormone can become active again. This occurs by interacting with normal gut bacteria. Gut bacteria have the ability to convert T3S and T3AC back into active T3! Therefore, normal thyroid hormone levels rely on a healthy gut environment and gut metabolism.
 Leaky Gut, Lipopolysaccarides and Thyroid Metabolism
Leaky Gut, Lipopolysaccarides and Thyroid Metabolism
We know chronic gut issues such as leaky gut and chronic gut infections will impact thyroid conversion of T4 to T3 as discussed. Gastrointestinal microorganisms release toxic compounds such as lipopolysaccharides (LPS) as part of bacterial metabolism which can impact thyroid physiology in several ways. Intestinal permeability (leaky gut) allows LPS to penetrate the intestinal barrier and enter circulation. Once in circulation, LPS has the potential to influence the thyroid at all levels of function, including reducing thyroid hormone levels, impacting conversion of T4 to T3, diminishing the expression of the thyroid receptor sites, increasing inactive RT3 levels, decreasing TSH and promoting AI thyroid conditions.[92-94]
In the subsequent articles in this series, we will describe the most common disorders that result from each of the primary organs of the GI tract, including the stomach, small intestine and large intestine.
References
63. Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008 Dec;66(3):487-95. doi: 10.1111/j.1574-6941.2008.00520.x. Epub 2008 Jun 4.
64. Focà A, Liberto MC, Quirino A, Matera G. Lipopolysaccharides: from Erinyes to Charites. Mediators Inflamm. 2012;2012:684274. doi: 10.1155/2012/684274. Epub 2012 May 14. Review.
65. McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008;28(4):281-99. Review.
66. Nahra R, Dellinger RP. Targeting the lipopolysaccharides: still a matter of debate? Curr Opin Anaesthesiol. 2008 Apr;21(2):98-104. doi:10.1097/ACO. 0b013e3282f5335c. Review.
67. Borella E, Palma L, et al. The body against self: autoinflammation and autoimmunity. Isr Med Assoc J. 2014 Oct;16(10):608-10. No abstract available.
68. Perricone C, Toubi E, Valesini G, Shoenfeld Y. Autoinflammation and autoimmunity: pathogenic, clinical, diagnostic and therapeutic aspects. Isr Med Assoc J. 2014 Oct;16(10): 601-4. No abstract available.
69. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009 Feb 1;65(3):263-7. Epub 2008 Aug 23
70. Irritable bowel syndrome, anxiety and depression: What are the links? J Clin Psychiatry. 2001;62 Suppl 8:38-45; discussion 46-7
71. Brain regulation of gastric acid secretion by neurogastrointestinal peptides. Peptides. 1981;suppl 2:51-5
72. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010 Mar;7(3):163-73. Epub 2010 Jan 26
73. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009 May;6(5):306-14. doi: 10.1038/nrgastro.2009.35. Review.
74. The role of paraventricular nucleus of hypothalamus in stress-ulcer formation in rats. Brain Res. 1997; 761(2):203-9.
75. Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5:604–615. [PubMed]
76. Heidi A. Jurgens, Rodney W. Johnson. Dysregulated Neuronal-Microglial Cross-Talk during Aging, Stress and Inflammation. Exp Neurol. 2012 January; 233(1): 40–48. Published online 2010 November 24. doi: 10.1016/j.expneurol.2010.11.014
77. Maes M, et al. The brain-gut barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008 Feb;29(1):117-24
78. Kimura K, et al. Concurrence of inflammatory bowel disease and multiple sclerosis. Mayo Clin Proc. Aug 2000; 75: 802-806
79. Geissler A. et al. Focal white-matter lesions in patients with inflammatory bowel disease. Lancet 1995;345:897-98
80. Neurodegeneration: a key factor in the ageing gut. Neurogastroenterol Motil. 2004 Apr; 16 Suppl 1:19-23
81. The role of neuroenteric hormones in intestinal infectious diseases. Curr Opin Gastroenterol. 2000 Nov; 16(6):536-40
82. The relationship between SNP of cholecystokinin gene and certain mental status and its forensic significance. Fa Yi Xue Za Zhi. 2008 Aug;24(4):284-7
83. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006 Mar;9(3):381-8. Epub 2/19/06
84. Neurotensin: role in psychiatric and neurological diseases. Peptides. 2006 Oct;27(10):2385-404. Epub 2006 Aug 4
85. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009 May;6(5):306-14
86. The probiotic bifidobacteria in infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008 Dec;43(2):164-74. Epub 2008 May 5.
87. W R Middleton. Thyroid hormones and the gut. Gut. 1971 February; 12(2): 172–177. 88. Ebert EC. The thyroid and the gut. J Clin Gastroenterol. 2010 Jul;44(6):402-6. doi: 10.1097/MCG.0b013e3181d6bc3e. Review.
89. Olga Yaylali, Suna Kirac, et al. Does Hypothyroidism Affect Gastrointestinal Motility? Gastroenterol Res Pract. 2009; 2009: 529802. Published online 2010 March 7. doi: 10.1155/ 2009/529802
90. W R Middleton. Thyroid hormones and the gut. Gut. 1971 February; 12(2): 172–177. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1411539/
90a. The effects of stress on gastric ulceration, T3, T4, reverse T3 and cortisol in neonatal foals. Equine Vet J. 1992; 24(1):37-40
90b. The effects of thyroid hormones on stress ulcer formation. Anz J Surg. 2002; 72(9):672-5
91. Johanna Laukkarinen, Juhani Sand, Isto Nordback. The Underlying Mechanisms: How Hypothyroidism Affects the Formation of Common Bile Duct Stones—A Review. HPB Surg. 2012; 2012: 102825. Published online 2012 September 19. doi: 10.1155/2012/ 102825
92. van der Poll T, Endert E, Coyle SM, Agosti JM, Lowry SF. Neutralization of TNF does not influence endotoxin-induced changes in thyroid hormone metabolism in humans. M J Physiol. 1999 Feb;276(2 Pt 2):R357-R362.
93. van der Poll T, Van Zee KJ, Endert E, et al. Interleukin-1 receptor blockade does not affect endotoxin-induced changes in plasma thyroid hormone and thyrotropin concentrations in man. J Clin Endocrinol Metabl. 1995 Apr;80(4):1341:1346.
94. Boelen A, Kwakkel J, Platvoet-ter Schiphorst M, et al. Interleukin-18, a proinflammatory cytokine, contributes to the pathogenesis of non-thyroidal illness mainly via the central part of the hypothalamus-pituitary-thyroid axis. Eur J Endocrinol. 2004 Oct;151(4):497-502.