 Health and maintenance of the gastrointestinal system is critical for successful aging and disorders of this system can result in various chronic diseases affecting multiple organs of the body. In this first segment of this series, we will review the critical functions of this system in the human body according to the most recent research.
Health and maintenance of the gastrointestinal system is critical for successful aging and disorders of this system can result in various chronic diseases affecting multiple organs of the body. In this first segment of this series, we will review the critical functions of this system in the human body according to the most recent research.
“Every living organism has to have two fundamental abilities: to bring in substances which provide energy and sustenance and to remove waste from the organism. We are exposed to innumerable substances in our environment simply by breathing, eating, drinking, working and playing. It is crucial that our bodies identify substances that are necessary to maintain health, absorb these substances into our body and circulate these throughout our circulatory system, where they can nourish all the cells of the bodies while protecting it from harmful substances from entering into circulation. It must also be able to eliminate waste and toxins which result from normal metabolism.”[1]
 Introduction
Introduction
Over a lifetime, a person will consume over 25 tons of food, providing the body with nutrients through the digestion and absorption process. During this process, the body must maintain protection from innumerable harmful external organisms and toxins that often accompany food. For example, in 1999 alone, over 1 billion pounds of pesticides were applied to U.S. crops and over 5.6 billion pounds were applied worldwide.[2] Pesticides are now a ubiquitous component of our environment and can be found within the home, in home gardens and our food we buy at the grocery store. While eating organic foods can greatly reduce our pesticide exposure, this does not guarantee elimination of pesticides. A 2001 review on toxin exposure indicated that the expense of treating environmentally-related conditions accounts for between $57 billion to $397 billion of the healthcare economy annually in the U.S and Canada.[2] Clearly, the ability to protect ourselves against these harmful compounds relies on a healthy digestive and absorptive system.
 Unfortunately, pesticides and pollutants are not the only harmful compounds that can accompany our food. Pathogens, such as harmful bacteria and parasites, can also be present in our food, causing various forms of stomach and gastrointestinal complaints. Then there are reactions to food, such as food allergies and sensitivities which are becoming increasingly common and growing at an alarming rate. Food allergies are estimated to affect 8% (almost 1 in 10) children worldwide.[4] Food allergies and intolerances account for a wide range of symptoms, from migraines and skin problems to neurological, inflammatory and gastrointestinal diso
Unfortunately, pesticides and pollutants are not the only harmful compounds that can accompany our food. Pathogens, such as harmful bacteria and parasites, can also be present in our food, causing various forms of stomach and gastrointestinal complaints. Then there are reactions to food, such as food allergies and sensitivities which are becoming increasingly common and growing at an alarming rate. Food allergies are estimated to affect 8% (almost 1 in 10) children worldwide.[4] Food allergies and intolerances account for a wide range of symptoms, from migraines and skin problems to neurological, inflammatory and gastrointestinal diso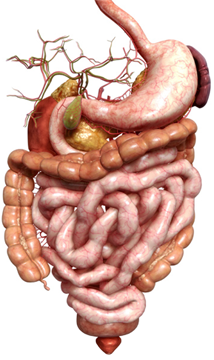 rders. Many of these reactions are related to intestinal permeability, discussed in Part 5 of the Successful Aging series.
rders. Many of these reactions are related to intestinal permeability, discussed in Part 5 of the Successful Aging series.
The human digestive system can be divided into the gastrointestinal (GI) tract and the accessory digestive organs. The organs of the GI tract include the oral cavity (mouth), pharynx (throat), esophagus, stomach, small intestine (divided into the duodenum, jejunum and ileum), large intestine (colon), rectum and anus. The accessory digestive organs include the salivary glands, liver, gall bladder and pancreas which aid in digestion and absorption of food and nutrients. As we will see, one of the primary functions of the GI tract is protection and this system protects our bodies from potentially harmful or toxic compounds in several ways.
Anatomy of the Gut
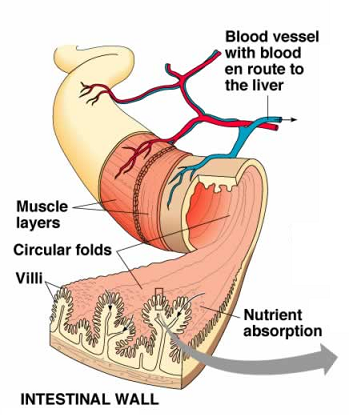
The GI tract, which is not unlike a long tube which extends from the mouth to the anus, is approximately 30 feet long. The gut wall is not smooth but is rough, with valleys and hills which increase the surface area of the gut. If you were to stretch out the surface area of the gut, it would cover the area of two tennis courts (3,000 sq. ft.)! How is this possible? 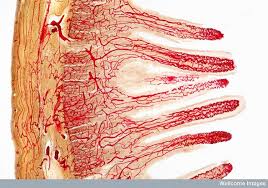 Because of microscopic finger-like projections called villi which increase the surface area and aid in absorption. The villi contain even smaller projections, called microvilli, which increase the surface area even further. We need this large surface area to maximize nutrient absorption, which takes place primarily in the small intestine. The primary functions of the digestive tract are motility, secretion, digestion, absorption, protection and elimination of waste.
Because of microscopic finger-like projections called villi which increase the surface area and aid in absorption. The villi contain even smaller projections, called microvilli, which increase the surface area even further. We need this large surface area to maximize nutrient absorption, which takes place primarily in the small intestine. The primary functions of the digestive tract are motility, secretion, digestion, absorption, protection and elimination of waste.
 Motility and Secretion
Motility and Secretion
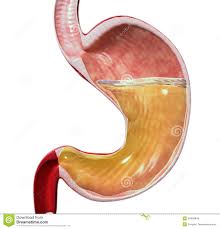
Motility refers to the movement of food through the GI tract through the process of ingestion (intake of food), mastication (chewing food and mixing with saliva), deglutition (swallowing of food) and peristalsis (the rhythmic wave-like contractions that move food through the GI tract). Secretion refers to the secretion of HCl (hydrochloric acid) and mucus in the stomach, bile (synthesized by the liver), bicarbonate and many pancreatic enzymes into the small intestine that work to digest (breakdown) all the different macronutrients that we consume and aid in absorption. A healthy stomach, for example, secretes two to three liters of gastric juice per day! If there are problems with secretion of these gastric enzymes (a common condition called hypochlorhydria), this can lead to a host of health problems, including gas, bloating, acid reflux or heartburn (called “GERD”), difficulty swallowing and stomach pain.
 Digestion
Digestion
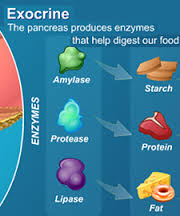
The role of the digestive process is to breakdown these food compounds into absorbable micronutrients and separate these individual nutrients from the complex food matrix that enters the body. This process begins with mastication (chewing of food into smaller pieces that can be swallowed) and continues as the ingested food passes through the esophagus to the stomach where the majority of protein digestion takes place by stomach acids such as hydrochloric acid and pepsin. Protein digestion continues in the small intestine due to the secretion of various pancreatic enzymes which enable further protein digestion and where most carbohydrate and lipid (fat) digestion takes place. Protein is broken into polypeptides, then amino acids which can then be absorbed in the small intestine. Carbohydrates get broken down by other pancreatic enzymes into polysaccharides, then into monosaccharides, such as glucose, which can then be absorbed in the small intestine.
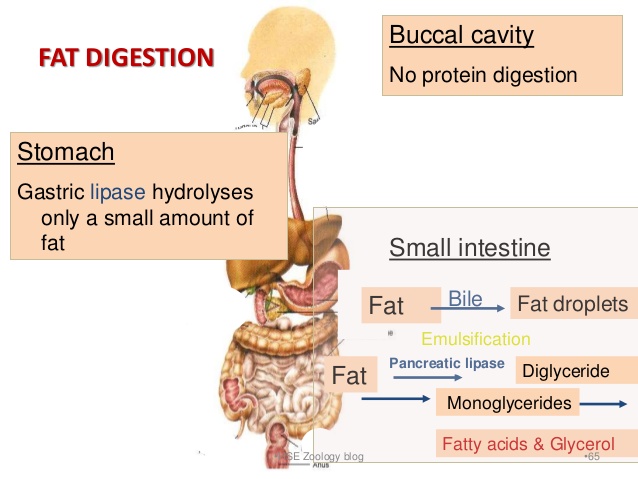 The digestion of lipids is a bit more complex. The arrival of lipids in the duodenum portion of the small intestine serves as a stimulus for the secretion of bile, produced by the liver and stored by the gall bladder. In a process called emulsification, bile salts contained in the bile, act to break up the fat droplets into smaller triglycerides. Pancreatic lipase enzymes further digest (break up) these triglycerides into free fatty acids and monoglycerides which then combine with micelles of bile salts, lecithin and cholesterol to form “mixed micelles” which can then be absorbed in the small intestine.
The digestion of lipids is a bit more complex. The arrival of lipids in the duodenum portion of the small intestine serves as a stimulus for the secretion of bile, produced by the liver and stored by the gall bladder. In a process called emulsification, bile salts contained in the bile, act to break up the fat droplets into smaller triglycerides. Pancreatic lipase enzymes further digest (break up) these triglycerides into free fatty acids and monoglycerides which then combine with micelles of bile salts, lecithin and cholesterol to form “mixed micelles” which can then be absorbed in the small intestine.
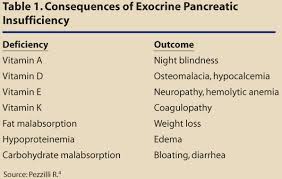 The secretion of adequate amounts of specific digestive enzymes is essential for healthy digestion and absorption of nutrients. If food compounds are not completely broken down into absorbable nutrients, then the body can suffer from malabsorption and malnutrition which are very common with the modern diet and lifestyle. Lack of any of these necessary digestive enzymes can have local and systemic effects (throughout the body). Pancreatic secretions often decrease with age or with conditions that place additional stress on the pancreas, such as insulin resistance, diabetes or chronic pancreatitis. Pancreatic secretions are also adversely affected by alcohol abuse, cystic fibrosis, gallstones and chronic inflammation. Pancreatic insufficiency requires oral replacement of pancreatic enzymes to aid in digestion.[5] Oral pancreatic enzymes have also been shown to reduce histamine secretion and symptoms associated with food allergies.[6]
The secretion of adequate amounts of specific digestive enzymes is essential for healthy digestion and absorption of nutrients. If food compounds are not completely broken down into absorbable nutrients, then the body can suffer from malabsorption and malnutrition which are very common with the modern diet and lifestyle. Lack of any of these necessary digestive enzymes can have local and systemic effects (throughout the body). Pancreatic secretions often decrease with age or with conditions that place additional stress on the pancreas, such as insulin resistance, diabetes or chronic pancreatitis. Pancreatic secretions are also adversely affected by alcohol abuse, cystic fibrosis, gallstones and chronic inflammation. Pancreatic insufficiency requires oral replacement of pancreatic enzymes to aid in digestion.[5] Oral pancreatic enzymes have also been shown to reduce histamine secretion and symptoms associated with food allergies.[6]
 Absorption
Absorption
Absorption of nutrients into the circulatory system takes place primarily in the small intestine but some nutrient absorption takes place in the stomach. Water-soluble nutrients (carbohydrates, amino acids and water-soluble vitamins) are absorbed in the epithelial cells of the intestinal villi (“finger-like” projections of GI lining) by both passive and active transport systems. These nutrients are secreted into blood capillaries where they enter circulation. After undergoing emulsification, as described above, fat-soluble nutrients (free fatty acids, monoglycerides and fat-soluble vitamins) are absorbed into the villi of the intestinal epithelial cells. Once these products enter the cells, triglycerides and phospholipids are resynthesized and combined with proteins to form particles that are secreted into the lymphatic capillaries of the intestinal villi. These absorbed lipids pass through the lymphatic system, eventually entering the venous circulation.
 How the Quality of Food Impacts Digestion and Absorption
How the Quality of Food Impacts Digestion and Absorption
The digestive process is influenced by the nature of the food consumed. Whole foods or minimally processed foods are generally digested more slowly than highly processed foods. This slows the release of nutrients and influences the rate at which absorption of both macro and micronutrients takes place. A diet high in sugar and fats will result in rapid absorption of glucose and fatty acids, creating increased demand upon the hormonal nutrient regulatory system and enzymes, including insulin, cortisol and insulin-like growth factor.[7] This is a major problem with the modern diet which relies heavily on fast food, processed foods and foods high in sugar. In addition, the selection of what foods to ingest, determines the quality of the diet (nutrient density) and the ultimate influence these nutrients have on physiological function. Let’s look at the important ways the GI tract provides protection to our bodies.
 Protection: Gut Barrier
Protection: Gut Barrier
“The primary functions of the gastrointestinal tract have traditionally been perceived to be limited to the digestion and absorption of nutrients and to electrolytes and water homeostasis. A more attentive analysis of the anatomic and functional arrangement of the gastrointestinal tract, however, suggests that another extremely important function of this organ is its ability to regulate the trafficking of macromolecules between the environment and the host through a barrier mechanism. Together with the gut-associated lymphoid tissue and the neuroendocrine network, the intestinal epithelial barrier, with its intercellular tight junctions, controls the equilibrium between tolerance and immunity to non-self antigens.”[8]
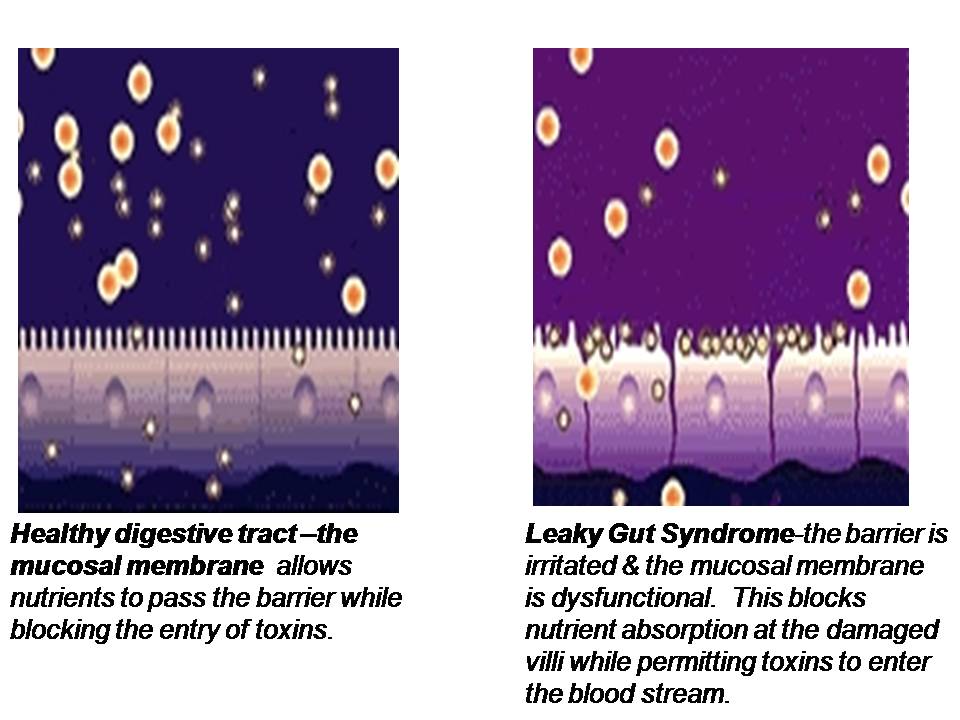
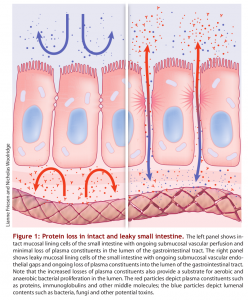 The lining of the GI tract is a mucosal membrane surface composed of epithelial cells that is the largest interface between our internal body and the external world. It covers over 3,000 square feet which is over 200 times the surface area of the skin! The GI mucosal membrane has the function of not only absorbing beneficial nutrients into the body but protecting the body against potentially damaging molecules and pathogenic organisms, such as undigested food particles, bacteria and other toxins that can accompany our food which should not be absorbed. Therefore, an important function of the GI mucosal lining is to provide a barrier between the internal body and the external world. Breakdown of this gut barrier leads to unwelcome molecules and toxic compounds potentially entering the body, a condition known as intestinal permeability or “leaky gut”. Leaky gut syndrome is commonly seen in intestinal inflammation, autoimmune conditions and food allergies and sensitivities.
The lining of the GI tract is a mucosal membrane surface composed of epithelial cells that is the largest interface between our internal body and the external world. It covers over 3,000 square feet which is over 200 times the surface area of the skin! The GI mucosal membrane has the function of not only absorbing beneficial nutrients into the body but protecting the body against potentially damaging molecules and pathogenic organisms, such as undigested food particles, bacteria and other toxins that can accompany our food which should not be absorbed. Therefore, an important function of the GI mucosal lining is to provide a barrier between the internal body and the external world. Breakdown of this gut barrier leads to unwelcome molecules and toxic compounds potentially entering the body, a condition known as intestinal permeability or “leaky gut”. Leaky gut syndrome is commonly seen in intestinal inflammation, autoimmune conditions and food allergies and sensitivities.
 Protection: Gastric Mucosa and Hydrochloric Acid
Protection: Gastric Mucosa and Hydrochloric Acid
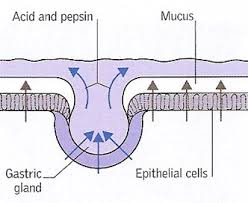
The GI mucosa includes the gastric epithelial layer which covers the stomach and protects it from the damaging stomach acid. This mucosa also protects the stomach from ingested toxins, drugs, alcohol and pathogens such as bacteria and viruses. The mucous gel that coats the stomach is comprised of phospholipids, secreted by gastric epithelial cells, called goblet cells. Parietal cells of the gastric lining secrete hydrochloric acid (HCl). Hydrochloric acid serves a protective function in the stomach as well. The high acidity of the stomach prevents bacteria from food and other sources from entering the body and inhabiting the GI tract, such as Helicobacter pylori (H. pylori).
 Protection: Gut-Associated Lymphoid Tissue (GALT)
Protection: Gut-Associated Lymphoid Tissue (GALT)
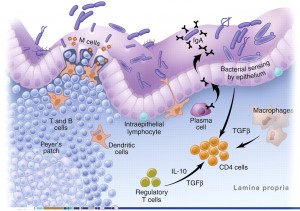
Another important protective role of the GI tract relates to the function of the gut-associated lymphoid tissue (GALT). Approximately 60% of the immune system, and more than 80% of the immunoglobulin-producing cells, are located within the mucosa of the GI tract. This complex immune system of the gut is called the gut-associated lymphoid tissue (GALT). The primary purpose of the GALT is to provide the first line of defense against foreign invaders such as food antigens (which elicit immune responses) and pathogenic bacteria and parasites. The GALT has two layers of defense to a foreign pathogen or antigen: localized secretory IgA (sIgA) antibody responses and the systemic IgE and IgG antibody responses.
 Protection: sIgA Cells
Protection: sIgA Cells
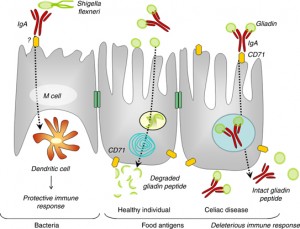
Secretory IgA (sIgA) cells are immune cells of the intestinal tract which protect us from bacteria, viruses and other pathogens and remove antigens (immune-stimulating compounds) before they can cross the mucosal barrier and reach circulation.[9,10] Low levels of sIgA have been associated with intestinal permeability and increasing uptake of food antigens, leading to atopic (allergic) reactions.[11] Antigens and foreign invaders which escape the sIgA surveillance can enter the mucosal layer where the GALT provides the second layer of defense. This involves the production of IgE and IgG antibodies which have a systemic immune response (throughout the circulatory system). In this immune response, antibodies are generated, cytokines are produced and the full immune response is engaged.
 Protection: Gut Microbiome
Protection: Gut Microbiome
Enteric microbiota (beneficial intestinal bacteria) which make up the gut microbiome play an important role in defense. It is estimated that there are approximately 100 trillion bacteria in the human digestive tract.[12-13] This means there are 10 times more bacterial cells in the gastrointestinal tract than there are cells in the human body! There are at least 1,000 different species of known bacteria with more than 3 million genes (approximately 150 times more bacterial genes than human genes)! [14] Accumulating evidence suggests that these commensal gut bacteria, that reside primarily in the large intestine, have a direct impact on promoting a healthy gut barrier, supporting various immune functions of defense and protecting from  a wide variety of chronic diseases related to multiple organs of the body.[15-21]
a wide variety of chronic diseases related to multiple organs of the body.[15-21]
“The study of gut microbiota is a rapidly moving field of research, and the impact of gut microbial communities on human health is widely perceived as one of the most exciting advancements in biomedicine in recent years. The gut microbiota plays a key role in digestion, metabolism and immune function, and has widespread impact beyond the gastrointestinal tract. Changes in the biodiversity of the gut microbiota are associated with far reaching consequences on host health and development.”[20]
 For example, the production of short-chain fatty acids (SCFA) by these bacteria promotes growth and differentiation of intestinal mucosal cells which protects against intestinal permeability and colon cancer.[22-25] These beneficial flora also play a major role in immune tolerance (as described above) to prevent against allergic diseases such as atopic skin disorders, eczema, and asthma.[26-31] High diversity of these gut flora appears to be protective of many chronic health conditions and loss of this diversity predisposes to a wide variety of chronic conditions such as inflammatory bowel disease [32-36], irritable bowel syndrome (IBS) [37], type 1 and type 2 diabetes [38-41], obesity [42-46], cancer [47-48], cardiovascular disease [49], bone loss [50], and urinary tract infections.[51] Recent research has focused on the gut-brain axis and has discovered an important connection of the microbiome to psychiatric conditions such as anxiety, depression, bipolar disorder, schizophrenia and autism. [52-61] Recent evidence has also shown a connection of the gut microbiota to neurodegenerative diseases as well, such as multiple sclerosis and Alzheimer’s disease.[62]
For example, the production of short-chain fatty acids (SCFA) by these bacteria promotes growth and differentiation of intestinal mucosal cells which protects against intestinal permeability and colon cancer.[22-25] These beneficial flora also play a major role in immune tolerance (as described above) to prevent against allergic diseases such as atopic skin disorders, eczema, and asthma.[26-31] High diversity of these gut flora appears to be protective of many chronic health conditions and loss of this diversity predisposes to a wide variety of chronic conditions such as inflammatory bowel disease [32-36], irritable bowel syndrome (IBS) [37], type 1 and type 2 diabetes [38-41], obesity [42-46], cancer [47-48], cardiovascular disease [49], bone loss [50], and urinary tract infections.[51] Recent research has focused on the gut-brain axis and has discovered an important connection of the microbiome to psychiatric conditions such as anxiety, depression, bipolar disorder, schizophrenia and autism. [52-61] Recent evidence has also shown a connection of the gut microbiota to neurodegenerative diseases as well, such as multiple sclerosis and Alzheimer’s disease.[62]
 Elimination of waste
Elimination of waste
The final important function of the GI tract is the elimination of waste which occurs primarily in the large intestine. Metabolism of nutrients in the GI tract leads to metabolic by-products which need to be eliminated by the body along with undigested food or toxins. Fecal excretion of the remnants and cellular debris of gut bacteria is also important. It is estimated there are 2-4 pounds of gut bacteria in the digestive tract which comprises 1-3% of our body weight. These organisms have their own natural life cycles and when they die, their cellular debris must be excreted. Enteric bacteria also produce many metabolites that must be detoxified and excreted as secondary metabolites.
In the next segment, we will look at various connections between the gastrointestinal system and several key organs of the body, including the thyroid, the immune system, the liver and the brain.
References
1. Jones, David, Editor in Chief. Textbook of Functional Medicine, Institute for Functional Medicine, 2006. p.189.
2. Jones, David, Editor in Chief. Textbook of Functional Medicine, Institute for Functional Medicine, 2006. p.189.
3. Muir T, Zegarac M. Societal costs of exposure to toxic substances: economic and health costs of four cases studies that are candidates for environmental causation. Environ Health Perspect. 2001;109(suppl 6):885-903.
4. Fogg MI, Spergel JM. Management of food allergies. Expert Opin Pharmacother. 2003;4:1025-1037.
5. Nakamura T, Takeuchi T, Tando Y. Pancreatic dysfunction and treatment options. Pancreas. 1998;16:329-336.
6. Raithel M, et al. Pancreatic enzymes: a new group of antiallergenic drugs? Inflamm Res. 2002;51(suppl 1):S13-S14.
7. Jones, David, Editor in Chief. Textbook of Functional Medicine, Institute for Functional Medicine, 2006. p.190.
8. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011 Jan;91(1):151-75. doi: 10.1152/physrev.00003.2008.
9. Lukaczer D. Secretory IgA and gastrointestinal barrier competence. Quarterly Rev Natural Med. 1996;Fall:227-229.
10. Albanese C, Smith S, Watkins S, et al. Effect of secretory IgA on transepithelial passage of bacteria across the intact ileum in vitro. J Am Coll Surg. 1994;179:679-688.
11. Ahmed T, Fuchs GJ. Gastrintestinal allergy to food: a review. J Diarrhoeal Dis Res. 1997;15:211-223.
12. Jean-Christophe Lagier, Matthieu Million, Perrine Hugon, Fabrice Armougom, Didier Raoult. Human Gut Microbiota: Repertoire and Variations. Front Cell Infect Microbiol. 2012; 2: 136. Prepublished online 2012 September 22. Published online 2012 November 2. doi: 10.3389/fcimb.2012.00136
13. Elizabeth A. Grice, Julia A. Segre. The Human Microbiome: Our Second Genome.
Annu Rev Genomics Hum Genet. Author manuscript; available in PMC 2013 June 6.
Published in final edited form as: Annu Rev Genomics Hum Genet. 2012; 13: 151–170. Published online 2012 June 6.
14. Qin J., Li R., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 464, 59–6510.1038/nature08821 [PMC free article] [PubMed] [Cross Ref]
15. Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012 Jan-Feb;95(1):50-60. Review.
16. Doré J, Blottière H. The influence of diet on the gut microbiota and its consequences for health. Curr Opin Biotechnol. 2015 Jan 20;32C:195-199. doi: 10.1016/j.copbio.2015.01.002. [Epub ahead of print] Review.
17. West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, Prescott SL; in-FLAMEMicrobiome Interest Group:; in-FLAME Microbiome Interest Group. The gut microbiota and inflammatory noncommunicable diseases: Associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015 Jan;135(1):3-13. doi: 10.1016/j.jaci.2014.11.012. Review.
18. Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease.
Curr Opin Gastroenterol. 2015 Jan;31(1):69-75. doi: 10.1097/MOG.0000000000000139.
19. Cénit MC, Matzaraki V, Tigchelaar EF, Zhernakova A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim Biophys Acta. 2014 Oct;1842(10):1981-1992. doi: 10.1016/j.bbadis.2014.05.023. Epub 2014 Jun 2. Review
20. Doré J, Simrén M, Buttle L, Guarner F. Hot topics in gut microbiota. United European Gastroenterol J. 2013 Oct;1(5):311-8. doi: 10.1177/2050640613502477.
21. Shanahan F. The colonic microbiota in health and disease. Curr Opin Gastroenterol. 2013 Jan;29(1):49-54. doi: 10.1097/MOG.0b013e32835a3493. Review.
22. Conlon MA, Bird AR. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients. 2014 Dec 24;7(1):17-44. Review.
23. O’Keefe SJ. Nutrition and colonic health: the critical role of the microbiota. Curr Opin Gastroenterol. 2008 Jan;24(1):51-8. Review.
24. Tomasello G, Tralongo P, Damiani P, Sinagra E, Di Trapani B, Zeenny MN, Hajj Hussein I, Jurjus A, Leone A. Dismicrobism in inflammatory bowel disease and colorectal cancer: Changes in response of colocytes. World J Gastroenterol. 2014 Dec 28;20(48):18121-18130. Review.
25. Lin XB, Farhangfar A, Valcheva R, Sawyer MB, Dieleman L, Schieber A, Gänzle MG, Baracos V. The role of intestinal microbiota in development of irinotecan toxicity and in toxicity reduction through dietary fibres in rats. PLoS One. 2014 Jan 14;9(1):e83644. doi: 10.1371/journal.pone.0083644. eCollection 2014.
26. Chistiakov DA, Bobryshev YV, Kozarov E, Sobenin IA, Orekhov AN. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Front Microbiol. 2015 Jan 13;5:781. doi: 10.3389/fmicb.2014.00781. eCollection 2014.
27. Tlaskalová-Hogenová H, Stepánková R, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004 May 15;93(2-3):97-108. Review.
28. Kim CH, Park J, Kim M. Gut microbiota-derived short-chain Fatty acids, T cells, and inflammation. Immune Netw. 2014 Dec;14(6):277-88. doi: 10.4110/in.2014.14.6.277. Epub 2014 Dec 22. Review.
29. West CE. Gut microbiota and allergic disease: new findings. Curr Opin Clin Nutr Metab Care. 2014 May;17(3):261-6. doi: 10.1097/MCO.0000000000000044. Review.
30. Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC.
Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014 Jun;44(6):842-50. doi: 10.1111/cea.12253.
31. Ismail IH, Oppedisano F, Joseph SJ, Boyle RJ, Licciardi PV, Robins-Browne RM, Tang ML. Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high-risk infants. Pediatr Allergy Immunol. 2012 Nov;23(7):674-81. doi: 10.1111/j.1399-3038.2012.01328.x. Epub 2012 Jul 26.
32. Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015 Jan;37(1):47-55. doi: 10.1007/s00281-014-0454-4. Epub 2014 Nov 25.
33. Hansen JJ, Sartor RB. Therapeutic Manipulation of the Microbiome in IBD: Current Results and Future Approaches. Curr Treat Options. Gastroenterol. 2015 Jan 18. [Epub ahead of print]
34. Cammarota G, Ianiro G, Cianci R, Bibbò S, Gasbarrini A, Currò D. The involvement of gut microbiota in inflammatory bowel disease pathogenesis: Potential for therapy. Pharmacol Ther. 2015 Jan 3. pii: S0163-7258(14)00235-6. doi: 10.1016/j.pharmthera.2014.12.006. [Epub ahead of print] Review.
35. Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014 Feb 7;20(5):1192-210. doi: 10.3748/wjg.v20.i5.1192. Review.
36. Tomasello G, Tralongo P, Damiani P, Sinagra E, Di Trapani B, Zeenny MN, Hajj Hussein I, Jurjus A, Leone A. Dismicrobism in inflammatory bowel disease and colorectal cancer: Changes in response of colocytes. World J Gastroenterol. 2014 Dec 28;20(48):18121-18130. Review.
37. Kassinen A., Krogius-Kurikka L., Makivuokko H., Rinttila T., Paulin L., Corander J. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007. 133,24–10.1053/j.gastro.2007.04. 005 [PubMed]
38. Gjymishka A, Coman RM, Brusko TM, Glover SC. Influence of host immunoregulatory genes, ER stress and gut microbiota on the shared pathogenesis of inflammatory bowel disease and Type 1 diabetes. Immunotherapy. 2013 Dec;5(12):1357-66. doi: 10.2217/imt.13.130. Review.
39. Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord. 2015 Jan 27. [Epub ahead of print]
40. Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol. 2014 Apr 29;5:190. doi: 10.3389/fmicb.2014.00190. eCollection 2014. Review.
41. Han JL, Lin HL. Intestinal microbiota and type 2 diabetes: From mechanism insights to therapeutic perspective. World J Gastroenterol. 2014 Dec 21;20(47):17737-17745. Review.
42. Ley R. E., Turnbaugh P. J., Klein S., Gordon J. I. (2006b). Microbial ecology: human gut microbes associated with obesity. Nature. 444, 1022–02310.1038/4441022a [PubMed]
43. Turnbaugh P. J., Backhed F., Fulton L., Gordon J. I. (2008). Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–22310.1016/j.chom.2008.02.015 [PMC free article] [PubMed]
44. Turnbaugh P. J., Hamady M., Yatsunenko T., Cantarel B. L., Duncan A., Ley R. E. A core gut microbiome in obese and lean twins. Nature. 457, 480–48410.1038/nature07540[PMC free article] [PubMed]
45. Turnbaugh P. J., Ley R. E., Mahowald M. A., Magrini V., Mardis E. R., Gordon J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–103110.1038/nature05414 [PubMed]
46. Santacruz A., Marcos A., Warnberg J., Marti A., Martin-Matillas M., Campoy C. (2009). Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 17, 1906–191510.1038/oby.2009.112 [PubMed]
47. Zitvogel L, Galluzzi L, Viaud S, Vétizou M, Daillère R, Merad M, Kroemer G. Cancer and the gut microbiota: An unexpected link. Sci Transl Med. 2015 Jan 21;7(271):271ps1. Review.
48. Ohtani N. Semin Immunopathol. Microbiome and cancer. 2015 Jan;37(1):65-72. doi: 10.1007/s00281-014-0457-1. Epub 2014 Nov 18.
49. Ettinger G, MacDonald K, Reid G, Burton JP. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014 Nov 2;5(6):719-28. doi: 10.4161/ 19490976.2014.983775.
50. Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2014 Dec 9. pii: S1043-2760(14)00201-X. doi: 10.1016/j.tem.2014.11.004. [Epub ahead of print] Review.
51. Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract-a role beyond infection. Nat Rev Urol. 2015 Jan 20. doi: 10.1038/nrurol.2014.361. [Epub ahead of print] Review.
52. Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014 Nov 12;34(46):15490-6. doi: 10.1523/ JNEUROSCI.3299-14.2014.
53. Gareau MG. Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol. 2014;817:357-71. doi: 10.1007/978-1-4939-0897-4_16. Review.
54. Sue Grenham, Gerard Clarke, John F. Cryan, Timothy G. Dinan. Brain–Gut–Microbe Communication in Health and Disease. Front Physiol. 2011; 2: 94. Prepublished online 2011 October 13. Published online 2011 December 7. doi: 10.3389/fphys.2011.00094
55. Rhee S. H., Pothoulakis C., Mayer E. A. (2009). Principles and clinical implications of the brain–gut–enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 6, 306–31410.1038/nrgastro.2009.35[PMC free article] [PubMed] [Cross Ref]
56. Dash S, Clarke G, Berk M, Jacka FN. The gut microbiome and diet in psychiatry: focus on depression. Curr Opin Psychiatry. 2015 Jan;28(1):1-6. doi: 10.1097/YCO.0000000000000117.
57. Slyepchenko A, Carvalho AF, Cha DS, Kasper S, McIntyre RS. Gut Emotions-Mechanisms of Action of Probiotics as Novel Therapeutic Targets for Depression and Anxiety Disorders. CNS Neurol Disord Drug Targets. 2014 Nov 30. [Epub ahead of print]
58. Fond G, Boukouaci W, et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol Biol (Paris). 2014 Nov 2. pii: S0369-8114(14)00163-1. doi: 10.1016/j.patbio.2014.10.003. [Epub ahead of print]Review.
59. Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res. 2014 Jul 14. pii: S0920-9964(14)00319-3. doi: 10.1016/j.schres.2014.06.027. [Epub ahead of print] Review.
60. Catanzaro R, Anzalone MG, et al. The gut microbiota and its correlations with the central nervous system disorders. Panminerva Med. 2014 Nov 12. [Epub ahead of print]
61. Finegold SM. Therapy and epidemiology of autism – clostridial spores as key elements. Med Hypotheses. 2008;70:508–511. doi:10.1016/j.mehy.2007.07.019. [PubMed]
62. Alam MZ, Alam Q, Kamal MA, Abuzenadah AM, Haque A. A possible link of gut microbiota alteration in type 2 diabetes and Alzheimer’s disease pathogenicity: an update. CNS Neurol Disord Drug Targets. 2014 Apr;13(3):383-90. Review.