 Gastroesophageal Reflux Disease (GERD)
Gastroesophageal Reflux Disease (GERD)
Gastroesophageal reflux disease (GERD) is an extremely prevalent condition in our society as evidenced by the constant television commercials bombarding us with ads for drugs that are supposed to solve all our heartburn problems and lead us to a better life. It has been estimated the prevalence of GERD symptoms in the general population may be up to 30% in the U.S.[125] but other studies show it could be as high as 34% to 40%.[126] Make no mistake about it. These are block-buster drugs that make the pharmaceutical companies billions of dollars annually. Proton-pump inhibitors (PPIs) are the third-highest selling class of drugs in the United States, with $13.9 billion in annual sales.[127] Sales of Nexium (the “purple pill” by Astra Zeneca) topped $6.1 billion in 2013 alone, the second highest prescribed medication in the U.S.[128]
 Is GERD the Result of Excess Acid Production?
Is GERD the Result of Excess Acid Production?
The symptoms of GERD include heartburn, chest pain and sour regurgitation. It is commonly believed to result from excess acid production in the stomach. This is what the drug companies want us to believe because this is the mechanism their drugs target. However, most of the medical literature on GERD indicates otherwise.
“First, excessive gastric acid secretion is not a major factor in GERD pathogenesis. Second, a substantial proportion of symptom-provoking reflux episodes are not caused by acid reflux.” [129]
 Excess Acid Production is Not the Underlying Cause of GERD
Excess Acid Production is Not the Underlying Cause of GERD
Let’s think about this. We know that as we age, gastric acid production decreases, along with pancreatic enzyme secretion.[130] If excess acid production was really the primary mechanism involved in GERD, we would expect to see young children at their peak of digestive function and enzyme secretion popping the purple pills to treat their heartburn! We would expect to see decreased incidence of GERD as people age. As we know, this is far from the reality and in fact, the opposite is the case. GERD is a much more common condition in the elderly, and the incidence of GERD increases, not decreases, as people age.[130] So obviously something else must be going on here that results in GERD. But of course, the drug companies won’t tell you this. And most doctors continue to prescribe PPIs to treat GERD telling their patients that they suffer from a problem of excess acid production. So what’s really going on in GERD?
 The Real Cause of GERD
The Real Cause of GERD
I will attempt to summarize a somewhat complex condition in the following section. For a more thorough explanation of GERD and the cause of GERD and bacterial overgrowth, please see my article: The Cause of GERD and Bacterial Overgrowth (coming soon)
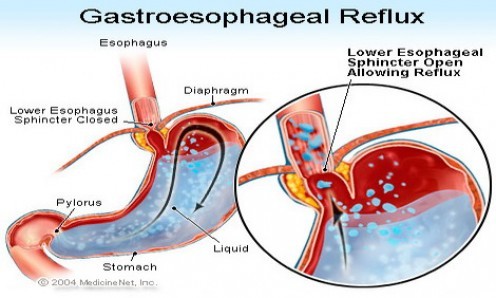
An important cause of GERD is related to failure of the valve mechanism which exists between the esophagus and the stomach, formed by the lower esophageal sphincter (LES) and the diaphragm, which normally prevents reflux of stomach contents into the esophagus.[135] A decrease in pressure of the LES can allow esophageal reflux of gastric contents. Some patients with GERD have a constantly weak, low-pressure LES, which permits reflux every time the pressure in the stomach exceeds the LES pressure. Periodic relaxation of the LES in normal individuals is called “transient lower esophageal sphincter relaxation” (TLESR). When it becomes more frequent and prolonged, TLESRs are believed to contribute significantly to reflux disease.[144]
“Recent studies have suggested that transient lower esophageal sphincter relaxation is  the main mechanism underlying gastroesophageal reflux.”[143]
the main mechanism underlying gastroesophageal reflux.”[143]
In patients with GERD, TLESRs account for the majority of reflux episodes.[145] Gastric distension (also known as “intra-abdominal pressure” or IAP) has been recognized as a major factor inducing TLESRs.[146-148]
“We conclude that gastric distention, by significantly increasing the rate of transient LES relaxations in both normal subjects and patients with gastroesophageal reflux disease, may contribute to the postprandial increase in gastroesophageal reflux.”[146]
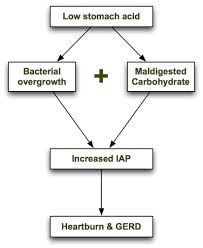
But the million-dollar question that few people are asking is: “What causes gastric distension or intra-abdominal pressure (IAP) in the first place?” There are several related conditions that lead to IAP, including hypochlorhydria, protein putrefaction, carbohydrate malabsorption and bacterial overgrowth. Let’s briefly take a look at these related conditions.
 Causes of GERD: Hypochlorhydria and Protein Putrefaction
Causes of GERD: Hypochlorhydria and Protein Putrefaction
It is well-documented in the scientific literature that indigestion of proteins in the intestines leads to a process known as protein putrefaction which can result in dyspepsia (indigestion), gas, bloating and diarrhea.[150,151] Protein putrefaction is caused by bacterial decomposition of undigested protein and results in production of foul-smelling odors.[152] This can also occur in the stomach.[153] Hydrochloric acid production and activity in the stomach is an important part of protein digestion. HCl partially digests protein so that it is more rapidly and efficiently digested by pancreatic enzymes in the small intestine. Decreased production of HCl (hypochlorhydria) or decreased pancreatic enzymes can lead to decreased protein digestion and increased protein putrefaction in the intestinal tract.[155] In addition, when hypochlorhydria is present, there is usually pancreatic enzyme deficiency present as well, since the acidity of the digested food stimulates the release of pancreatic enzymes. This then leads to carbohydrate malabsorption.
“Protein putrefaction is often found alongside that of carbohydrate (malabsorption), though the latter is always the more marked.”[155]
 Causes of GERD: Carbohydrate Malabsorption and Bacterial Overgrowth
Causes of GERD: Carbohydrate Malabsorption and Bacterial Overgrowth
In a book called, “Heartburn Cured: The Low-Carb Miracle”, microbiologist Norm Robillard, Ph.D., postulates that carbohydrate malabsorption leads to an increase in intra-abdominal pressure (IAP) resulting in acid reflux.[158] Dr. Robillard points out that intestinal bacteria primarily feed on carbohydrates and that these bacteria will ferment any carbohydrate that is not absorbed. Bacterial fermentation leads to excess gas production. The resulting gas increases IAP, which is the driving force behind loss of LES function, acid reflux and GERD.
 When stomach acid is sufficient and carbohydrates are consumed in moderation, they are properly broken down into glucose and rapidly absorbed in the small intestine before they can be fermented by small intestinal bacteria. However, if stomach acid is insufficient or carbohydrates are consumed in excess, some of the carbohydrate can escape absorption and become available for fermentation by intestinal bacteria. Carbohydrate malabsorption therefore leads to an increase in bacterial fermentation and increased gas production.
When stomach acid is sufficient and carbohydrates are consumed in moderation, they are properly broken down into glucose and rapidly absorbed in the small intestine before they can be fermented by small intestinal bacteria. However, if stomach acid is insufficient or carbohydrates are consumed in excess, some of the carbohydrate can escape absorption and become available for fermentation by intestinal bacteria. Carbohydrate malabsorption therefore leads to an increase in bacterial fermentation and increased gas production.
Dr. Robillard cites a study by Suarez and Levitt, showing that 30 grams of carbohydrate that escapes absorption in a day could produce more than 10,000 mL (ten liters) of hydrogen gas! Additionally, increased undigested carbohydrate in the stomach and intestines allows for bacterial overgrowth since these intestinal bacteria will proliferate with large amounts of undigested food. So his solution to GERD and bacterial overgrowth is simple: decrease carbohydrate intake!
 Hypochlorhydria: The Primary Cause of Carbohydrate Malabsorption, Bacterial Overgrowth and GERD?
Hypochlorhydria: The Primary Cause of Carbohydrate Malabsorption, Bacterial Overgrowth and GERD?
Stomach acid (HCl) is primarily involved in protein digestion, however, HCl content can also affect carbohydrate digestion. HCl supports the digestion and absorption of carbohydrates by stimulating the release of pancreatic stimuli such as CCK and secretin, which then stimulate pancreatic secretion of enzymes. If the acidity of the intestinal contents is too low (due to insufficient stomach acid), this can affect the secretion of pancreatic enzymes and can affect carbohydrate digestion. Interestingly, human trials examining the effects of HCl on carbohydrate digestion are unavailable in a thorough search of the literature. However, in vitro studies have shown that carbohydrate digestion of rice, starches and vegetables, is improved in the presence of increased hydrochloric acid (HCl). [167-169] Human studies have also shown improved fat digestion and absorption in the presence of HCl.[170]
 Does HCl Supplementation Decrease Gastric Distension and GERD?
Does HCl Supplementation Decrease Gastric Distension and GERD?
It’s really quite shocking how few studies have been done to investigate the benefits of HCl supplementation in the treatment of GERD. Why is this? The most probable explanation is that there is no profit in carrying out this type of study since drug companies cannot patent HCl. There was one small study performed which examined whether the symptoms of gastric distension (IAP) in a group of 9 people were influenced by gastric infusion of HCl. Not surprisingly, the study concluded that sensations of gastric distension were reduced by adding HCl into the stomach in the study group.[171] A more interesting study, much larger in size and involving over 350 people with GERD, divided the participants into 2 groups. The first group (A) received a supplement that contained a combination of betaine HCl, melatonin, l-tryptophan, vitamins B6, B12 and folic acid and methionine. The other group (B) received 20 mg of omeprazole (PPI). Here’s what the study concluded:
“All patients of the group A (100%) reported a complete regression of symptoms after 40 days of treatment. On the other hand, 115 subjects (65.7%) of the omeprazole (PPI) reported regression of symptoms in the same period. There was a statistically significant difference between the groups (P < 0.05). This formulation promotes regression of GERD symptoms with no significant side effects.”[172]
 Conventional Treatment of GERD
Conventional Treatment of GERD
Conventional treatment of GERD relies on acid-suppressing medications, primarily PPIs (proton-pump inhibitors) such as omeprazole. However, as discussed, there are several problems with using PPIs or other acid-suppressing medication to treat GERD. First, PPIs do not address the underlying cause of GERD (LES dysfunction due to increased intra-abdominal pressure secondary to hypochlorhydria and carbohydrate malabsorption) and therefore are not very effective in the management of GERD. Second, there are many adverse effects associated with PPIs. Let’s start by briefly reviewing the research on the efficacy of PPIs in the treatment of GERD. We will then take a close look at the research on the various adverse effects associated with this class of medications.
 PPIs Are Not Effective Against GERD
PPIs Are Not Effective Against GERD
Proton-pump inhibitors (PPIs) are the strongest class of acid-suppressing medications available. They are very effective in inhibiting the production of acid in the stomach. However, as we have seen, excess acid production is not the cause of GERD. Since we now know the cause of GERD is insufficient production of HCl leading to carbohydrate malabsorption, bacterial fermentation and increased intra-abdominal pressure causing dysfunction of the LES, we can see how acid-suppressing medications, such as PPIs, do nothing to address the underlying cause of GERD or reduce the incidence of GERD.  This has been shown to be the case by several large studies.[135,173,174] People continue to have the same underlying problems of GERD while on PPIs. The only difference is there is less acid content available in their refluxate to irritate the esophagus.[135,173,174] And like most drugs, once the medication is stopped, the original condition returns.
This has been shown to be the case by several large studies.[135,173,174] People continue to have the same underlying problems of GERD while on PPIs. The only difference is there is less acid content available in their refluxate to irritate the esophagus.[135,173,174] And like most drugs, once the medication is stopped, the original condition returns.
“The simultaneous use of intra-esophageal impedance and pH measurement of acid and non-acid gastroesophageal reflux has clearly shown that treatment with PPIs only changes the pH of the refluxate, without stopping reflux through a functionally or mechanically incompetent LES. Vela et al have shown that during treatment with omeprazole, postprandial reflux still occurs but it becomes predominantly non-acid.”[135]
 Risks Associated with PPIs
Risks Associated with PPIs
“Emerging data illustrate the potential risks associated with both short-and long-term PPI therapy, including Clostridium difficile–associated diarrhea, community-acquired pneumonia, osteoporotic fracture, vitamin B12 deficiency, and inhibition of antiplatelet therapy. Due to these associations, it is recommended that clinicians assess the continuing need for PPI therapy and use the lowest possible dose to achieve the desired therapeutic goals.”[176]
“…mounting data demonstrate concerns about the long-term use of PPIs…the collective body of information overwhelmingly suggests an increased risk of infectious complications and nutritional deficiencies.”[175]
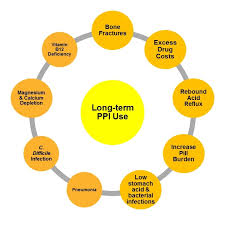 “The risk of pneumonia was increased 27-39% in short-term use of PPIs in three meta-analyses. C. difficile infections were also associated with the use of PPIs (odds ratio: 2.15; 95% CI: 1.81-2.55; p < 0.00001). This effect appears to be dose related. The U.S. FDA has recently issued a warning regarding fractures and the impaired magnesium absorption associated with the use of PPIs. Thrombocytopenia, iron deficiency, vitamin B12 deficiency, rhabdomyolysis and acute interstitial nephritis have also been reported with the use of PPIs.
“The risk of pneumonia was increased 27-39% in short-term use of PPIs in three meta-analyses. C. difficile infections were also associated with the use of PPIs (odds ratio: 2.15; 95% CI: 1.81-2.55; p < 0.00001). This effect appears to be dose related. The U.S. FDA has recently issued a warning regarding fractures and the impaired magnesium absorption associated with the use of PPIs. Thrombocytopenia, iron deficiency, vitamin B12 deficiency, rhabdomyolysis and acute interstitial nephritis have also been reported with the use of PPIs.
There is mounting evidence that PPIs are associated with serious adverse effects. Practitioners should be vigilant and counsel patients accordingly.”[177]
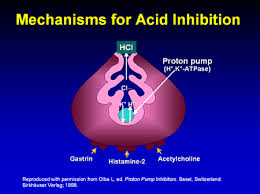 How Do Proton Pump Inhibitors (PPIs) Work?
How Do Proton Pump Inhibitors (PPIs) Work?
PPIs act by strongly inhibiting the enzyme in parietal cells that allows for acid production in the stomach.[178] They represent the most powerful class of acid-suppressing medications ever developed. Since stomach acid has three primary functions in the stomach (ie: prevent bacterial infection, aid in digestion of protein and aid in nutrient absorption) it’s not surprising that the risks associated with the use of PPIs or other acid-suppressing medications would be related to these critical functions of stomach acid. Studies have shown both short- and long-term use of these medications have been associated with various side effects, such as decreased absorption of vitamins and minerals, such as calcium, vitamin B12, iron and magnesium and increased risk of bone fractures.[179-182]
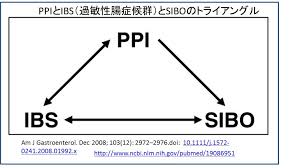 PPIs are recently being associated with increased risk of bacterial overgrowth in the small intestine (SIBO) due to their acid-suppressing effects, related to the important role that acid plays in preventing bacterial infection and preventing dysbiosis (imbalance of gut bacteria). [183-186] They have also been shown to inhibit digestion of proteins and cause food allergy in an animal model study.[187] PPIs are now routinely used in infants and children who suffer from vomiting attributed to acid reflux but not without adverse effects. One long-term study found that adverse effects have been reported in 34% of children
PPIs are recently being associated with increased risk of bacterial overgrowth in the small intestine (SIBO) due to their acid-suppressing effects, related to the important role that acid plays in preventing bacterial infection and preventing dysbiosis (imbalance of gut bacteria). [183-186] They have also been shown to inhibit digestion of proteins and cause food allergy in an animal model study.[187] PPIs are now routinely used in infants and children who suffer from vomiting attributed to acid reflux but not without adverse effects. One long-term study found that adverse effects have been reported in 34% of children  treated with PPIs and include headaches, diarrhea, nausea and constipation.[188] PPIs have also been associated with more severe infections, including hospital-acquired life-threatening infections in immune-suppressed infants and children.
treated with PPIs and include headaches, diarrhea, nausea and constipation.[188] PPIs have also been associated with more severe infections, including hospital-acquired life-threatening infections in immune-suppressed infants and children.
“Acid suppression may place immune deficient infants and children or those with indwelling catheters, at risk for the development of lower respiratory infections and nosocomial sepsis.”[188]
 Summary of GERD and the Treatment of GERD with PPIs:
Summary of GERD and the Treatment of GERD with PPIs:
As the current literature makes clear, excess acid production is not the underlying cause of GERD. Recent research has found that GERD results from dysfunction of the lower esophageal sphincter (LES), which allows gastric contents (including stomach acids) to reflux into the esophagus, causing the symptoms associated with acid reflux. The cause of dysfunction of the LES appears to be related to increased intra-abdominal pressure (IAP), which results from incomplete digestion of protein and carbohydrates due to insufficient production of stomach acids. Stomach acid is important in protein digestion and also aids in stimulation of the pancreas to secrete digestive enzymes which are used in carbohydrate digestion and absorption.
 Dr. Robillard’s pioneering work supports the concept that incomplete carbohydrate digestion and absorption causes increased bacterial fermentation in the intestines leading to increased gas and IAP. According to his research, this is the cause of GERD, which is supported by the literature on this topic, pointing to IAP as the cause of GERD. Acid-suppressing medications such as PPIs have no impact on the frequency or severity of esophageal reflux. The only impact that PPIs have on the symptoms of GERD is making the contents of the refluxate less acidic, thereby decreasing symptoms associated with stomach acid entering the esophagus. However, there are many adverse effects associated with PPI use, including increased risk of bacterial infection, decreased absorption of various vitamins and minerals leading to nutritional deficiencies and osteoporosis-related bone fractures.
Dr. Robillard’s pioneering work supports the concept that incomplete carbohydrate digestion and absorption causes increased bacterial fermentation in the intestines leading to increased gas and IAP. According to his research, this is the cause of GERD, which is supported by the literature on this topic, pointing to IAP as the cause of GERD. Acid-suppressing medications such as PPIs have no impact on the frequency or severity of esophageal reflux. The only impact that PPIs have on the symptoms of GERD is making the contents of the refluxate less acidic, thereby decreasing symptoms associated with stomach acid entering the esophagus. However, there are many adverse effects associated with PPI use, including increased risk of bacterial infection, decreased absorption of various vitamins and minerals leading to nutritional deficiencies and osteoporosis-related bone fractures.  Worse than that, by suppressing acid production, PPIs lead to decreased protein and carbohydrate digestion and increased risk of malabsorption, intra-abdominal pressure and GERD. One editorial entitled “Evidence That Proton-Pump Inhibitor Therapy Induces the Symptoms it is Used to Treat” commented on an article published in a British gastroenterology journal which came to this very conclusion:
Worse than that, by suppressing acid production, PPIs lead to decreased protein and carbohydrate digestion and increased risk of malabsorption, intra-abdominal pressure and GERD. One editorial entitled “Evidence That Proton-Pump Inhibitor Therapy Induces the Symptoms it is Used to Treat” commented on an article published in a British gastroenterology journal which came to this very conclusion:
“Treating gastroesophageal reflux disease with profound acid inhibition will never be ideal because acid secretion is not the primary underlying defect. Acid secretion is normal in most patients with reflux disease and acid inhibitory therapy makes it abnormally low. It is never ideal to treat one abnormality by creating another, as was the case for many years with management of ulcer disease before the disco- very of H. pylori infection.  The pathophysiology of acid reflux concerns the dysfunction of the gastroesophageal barrier and research needs to refocus on ways of restoring its competence rather than merely suppressing gastric acid secretion.”[238]
The pathophysiology of acid reflux concerns the dysfunction of the gastroesophageal barrier and research needs to refocus on ways of restoring its competence rather than merely suppressing gastric acid secretion.”[238]
 Clinical Management of GERD in Functional Medicine
Clinical Management of GERD in Functional Medicine
Many functional medicine doctors have found that supplementation with betaine HCl and pepsin has been extremely helpful in managing GERD and its associated symptoms. Some people will experience a “burning” sensation in the stomach when they supplement with HCl. I attribute this to irritation or thinning of the gastric lining that protects the stomach from the acidic HCl. In many cases, when we address the irritated gastric lining, they are able to tolerate HCl supplementation. I believe the evidence is strong enough to warrant the use of HCl and pepsin and the clinical response with minimal side effects further justifies its use in the management of GERD. In addition, zinc carnosine has been shown to be helpful in managing a wide range of gastrointestinal conditions.[239]  Zinc has been shown to improve the integrity of gastrointestinal epithelial tight junctions and barrier function and have protective effects of the stomach lining against gastric HCl.[239,240]
Zinc has been shown to improve the integrity of gastrointestinal epithelial tight junctions and barrier function and have protective effects of the stomach lining against gastric HCl.[239,240]
In the next segment, we will take a closer look at the adverse effects associated with PPIs according to the most current literature. In the following segment, we will continue to examine the most common disorders that result from dysfunction of the stomach, including gastric (peptic) ulcers and duodenal ulcer disease.
References
125. Mohammad Bashashati, Reza A Hejazi, Christopher N Andrews, Martin A Storr. Gastroesophageal reflux symptoms not responding to proton pump inhibitor: GERD, NERD, NARD, esophageal hypersensitivity or dyspepsia? Can J Gastroenterol Hepatol. 2014 June; 28(6): 335–341.
126. Huihui Sun, Ying Chen, et al. Abnormal activity of default mode network in GERD patients. BMC Neurosci. 2013; 14: 69. Published online 2013 July 11. doi: 10.1186/1471-2202-14-69
127. http://www.aafp.org/news/clinical-care-research/20100601ppifracture.html
128. http://www.medscape.com/viewarticle/820011
129. Guy Boeckxstaens, Hashem B El-Serag, André J P M Smout, Peter J Kahrilas. Symptomatic reflux disease: the present, the past and the future. Gut. 2014 July; 63(7): 1185–1193. Published online 2014 March 7. doi: 10.1136/gutjnl-2013-306393
130. Britton E, McLaughlin JT. Ageing and the gut. Proc Nutr Soc. 2013 Feb;72(1):173-7. doi: 10.1017/S0029665112002807. Epub 2012 Nov 12. Review.
131. Moraes-Filho J, Cecconello I, Gama-Rodrigues J, Castro L, Henry MA, Meneghelli UG, Quigley E. Brazilian consensus on gastroesophageal reflux disease: proposals for assessment, classification, and management. Am J Gastroenterol. 2002;97:241–248. [PubMed]
132. Sabine Roman, John E Pandolfino. Environmental–life style related factors. Best Pract Res Clin Gastroenterol. Author manuscript; available in PMC 2013 February 26. Published in final edited form as: Best Pract Res Clin Gastroenterol. 2010 December; 24(6): 847–859. doi: 10.1016/j.bpg.2010.09.010
133. Fatin R. Polat, Sabriye Polat. The Effect of Helicobacter Pylori on Gastroesophageal Reflux Disease. JSLS. 2012 Apr-Jun; 16(2): 260–263. doi: 10.4293/108680812X134279823-76860
134. B T Johnston, S A Lewis, A H Love. Psychological factors in gastro-oesophageal reflux disease. Gut. 1995 April; 36(4): 481–482.
135. Fernando A Herbella, Marco G Patti. Gastroesophageal reflux disease: From pathophysiology to treatment. World J Gastroenterol. 2010 August 14; 16(30): 3745–3749. Published online 2010 August 14. doi: 10.3748/wjg.v16.i30.3745
136. Tack J, Koek G, Demedts I, Sifrim D, Janssens J. Gastroesophageal reflux disease poorly responsive to single-dose proton pump inhibitors in patients without Barrett’s esophagus: acid reflux, bile reflux, or both? Am J Gastroenterol. 2004;99:981–988.[PubMed]
137. Herbella FA, Nipominick I, Patti MG. From sponges to capsules. The history of esophageal pH monitoring. Dis Esophagus. 2009;22:99–103. [PubMed]
138. Diener U, Patti MG, Molena D, Fisichella PM, Way LW. Esophageal dysmotility and gastroesophageal reflux disease. J Gastrointest Surg. 2001;5:260–265. [PubMed]
139. Patti MG, Perretta S. Gastro-oesophageal reflux disease: a decade of changes. Asian J Surg. 2003;26:4–6.[PubMed]
140. Meneghetti AT, Tedesco P, Damani T, Patti MG. Esophageal mucosal damage may promote dysmotility and worsen esophageal acid exposure. J Gastrointest Surg. 2005;9:1313–1317. [PubMed]
141. Kahrilas PJ. Anatomy and physiology of the gastroesophageal junction. Gastroenterol Clin North Am. 1997;26:467–486. [PubMed]
142. F De Giorgi, M Palmiero, I Esposito, F Mosca, R Cuomo. Pathophysiology of gastro-oesophageal reflux disease. Acta Otorhinolaryngol Ital. 2006 October; 26(5): 241–246.
143. Hirsch DP1, Tytgat GN, Boeckxstaens GE. Transient lower oesophageal sphincter relaxations–a pharmacological target for gastro-oesophageal reflux disease? Aliment Pharmacol Ther. 2002 Jan;16(1):17-26.
144. Kessing BF1, Conchillo JM, Bredenoord AJ, Smout AJ, Masclee AA. Review article: the clinical relevance of transient lower oesophageal sphincter relaxations in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2011 Mar;33(6):650-61. doi: 10.1111/j.1365-2036.2010.04565.x. Epub 2011 Jan 10.
145. Mittal RK, McCallum RW. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology. 1988;95:593-9. [PubMed]
146. Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distension: a mechanism for postprandial gastroesophageal reflux. Gastroenterology 1985;89:779-84. [PubMed]
147. Straathof JW1, van Veen MM, Masclee AA. Provocation of transient lower esophageal sphincter relaxations during continuous gastric distension. Scand J Gastroenterol. 2002 Oct;37(10):1140-3.
148. Scheffer RCH, Akkermans LMA, Bais JE, Roelofs JM, Smout AJ, Gooszen HG. Elicitation of transient lower oesophageal sphincter relaxations in response to gastric distension and meal ingestion. Neurogastroenterol Motil 2002; 14: 647–55.
149. C P Barham, D C Gotley, R Miller, A Mills, D Alderson. Pressure events surrounding oesophageal acid reflux episodes and acid clearance in ambulant healthy volunteers. Gut. 1993 April; 34(4): 444–449.
150. José M. Rosell. Studies in the Pathology of Digestion: I. Intestinal Fermentation and Putrefaction. Can Med Assoc J. 1929 July; 21(1): 42–46.
151. José M. Rosell. Studies in Digestive Pathology: II. The Mechanism of Fermentation and Putrefaction in the Intestines. Can Med Assoc J. 1929 August; 21(2): 161–168.
Appl Microbiol Biotechnol. 2014 Mar;98(6):2779-87. doi: 10.1007/s00253-013-5271-5. Epub 2013 Oct 10.
152. http://en.wikipedia.org/wiki/Putrefaction
153. W. Hale White, M.D. The Royal Society of Medicine, March 10, 1913. A discussion on alimentary toxaemia; its sources, consequences, and treatment. General Survey.
154. Zajac, P; Holbrook, A; Super, ME; Vogt, M (March–April 2013). An overview: Current clinical guidelines for the evaluation, diagnosis, treatment, and management of dyspepsia. Osteopathic Family Physician 5 (2): 79–85.doi:10.1016/j.osfp.2012.10.005.
155. Physiology And Pathology With Its Applications To Practical Medicine. Ry Carl Von Noorden Professor of The First University Medical Clinic, Vienna Vol. II.— The Pathology Of Metabolism. By Carl Von Noorden, Fr. Kraus, Ad. Schmidt, W. Weintraud, M. Matthes, H. Strauss.
156. https://www.nfpt.com/blog/biochemistry-nutrition/
157. Li JV, Ashrafian H, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011 Sep;60(9):1214-23. doi: 10.1136/gut.2010.234708. Epub 2011 May 14.
158. Heartburn Cured: The Low Carb Miracle. Norm Robillard, Ph.D., 2005. www.heartburncured.com
159. Pehl C1, Pfeiffer A, Wendl B, Stellwag B, Kaess H. Effect of erythromycin on postprandial gastroesophageal reflux in reflux esophagitis. Dis Esophagus. 1997 Jan;10(1):34-7.
160. Pennathur A1, Tran A, Cioppi M, Fayad J, Sieren GL, Little AG. Erythromycin strengthens the defective lower esophageal sphincter in patients with gastroesophageal reflux disease. Am J Surg. 1994 Jan;167(1):169-72; discussion 172-3.
161. Austin GL, Thiny MT, Westman EC, Yancy WS Jr, Shaheen NJ. A very low-carbohydrate diet improves gastroesophageal reflux and its symptoms. Dig Dis Sci. 2006 Aug;51(8):1307-12. Epub 2006 Jul 27.
162. Yancy WS Jr, Provenzale D, Westman EC. Improvement of gastroesophageal reflux disease after initiation of a low-carbohydrate diet: five brief case reports. Altern Ther Health Med. 2001 Nov-Dec;7(6):120, 116-9.
163. Yoshihisa Urita, Toshiyasu Watanabe, et al. Extensive Atrophic Gastritis Increases Intraduodenal Hydrogen Gas. Gastroenterol Res Pract. 2008; 2008: 584929. Published online 2008 June 16. doi: 10.1155/2008/584929
164. Diagnosis and management of small intestinal bacterial overgrowth. Nutr Clin Pract. 2013 Jun;28(3):289-299.
165. Theisen J1, Nehra D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterialovergrowth and deconjugation of bile acids. J Gastrointest Surg. 2000 Jan-Feb;4(1):50-4.
166. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013 May;11(5):483-490.
167. Li H, Zhu Y, Jiao A, Zhao J, Chen X, Wei B, Hu X, Wu C, Jin Z, Tian Y. Impact of α-amylase combined with hydrochloric acid hydrolysis on structure and digestion of waxy rice starch. Int J Biol Macromol. 2013 Apr;55:276-81. doi: 10.1016/j.ijbiomac.2013.01.021. Epub 2013 Jan 26.
168. Vallejo F, Gil-Izquierdo A, Pérez-Vicente A, García-Viguera C. In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J Agric Food Chem. 2004 Jan 14;52(1):135-8.
169. Ginette Doué, Micaël Bédikou, Gisèle Koua, Rose-Monde Mégnanou, Sébastien Niamké. Enzymatic and acid conversion of new starches from improved orphan crops: prospects for renewable materials uses in food and non-food industries. Springerplus. 2014; 3: 498. Published online 2014 September 3. doi: 10.1186/2193-1801-3-498
170. Parker Vanamee, Walter Lawrence, Jr., Sam Levin, Ann S. Peterson, Henry T. Randall. Further Observations on Postgastrectomy Steatorrhea: The Effect of High Carbohydrate Intake and of Hydrochloric Acid Administration on Fat Absorption. Ann Surg. 1959 September; 150(3): 517–527.
171. Coffin B1, Chollet R, Flourié B, Lémann M, Franchisseur C, Rambaud JC, Jian R. Intraluminal modulation of gastric sensitivity to distension: effects of hydrochloric acid and meal. Am J Physiol Gastrointest Liver Physiol. 2001 May;280(5):G904-9.
172. Pereira Rde S. Regression of gastroesophageal reflux disease symptoms using dietary supplementation with melatonin, vitamins and aminoacids: comparison with omeprazole. J Pineal Res. 2006 Oct;41(3):195-200.
173. Tamhankar AP1, Peters JH, Portale G, Hsieh CC, Hagen JA, Bremner CG, DeMeester TR. Omeprazole does not reduce gastroesophageal reflux: new insights using multichannel intraluminal impedance technology. J Gastrointest Surg. 2004 Nov;8(7):890-7; discussion 897-8.
174. Tack J1, Koek G, Demedts I, Sifrim D, Janssens J. Gastroesophageal reflux disease poorly responsive to single-dose proton pump inhibitors in patients without Barrett’s esophagus: acid reflux, bile reflux, or both? Am J Gastroenterol. 2004 Jun;99(6):981-8.
175. Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122:896–903.
176. Joel J. Heidelbaugh, Kathleen L. Goldberg, John M. Inadomi. Adverse Risks Associated With Proton Pump Inhibitors: A Systematic Review. Gastroenterol Hepatol (N Y) 2009 October; 5(10): 725–734.
177. Wilhelm SM1, Rjater RG, Kale-Pradhan PB. Perils and pitfalls of long-term effects of proton pump inhibitors. Expert Rev Clin Pharmacol. 2013 Jul;6(4):443-51. doi: 10.1586/17512433.2013.811206.
178. John Calam, J H Baron. Pathophysiology of duodenal and gastric ulcer and gastric cancer. BMJ. 2001 October 27; 323(7319): 980–982.
179. Tetsuhide Ito, Robert T. Jensen. Association of Long-term Proton Pump Inhibitor Therapy with Bone Fractures and effects on Absorption of Calcium, Vitamin B12, Iron, and Magnesium. Curr Gastroenterol Rep. December; 12(6): 448–457. doi: 10.1007/s11894-010-0141-0
180. Laura E. Targownik, Lisa M. Lix, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008 August 12; 179(4): 319–326. doi 10.1503/cmaj .071330
181. Katz PO1, Zavala S. Proton pump inhibitors in the management of GERD. J Gastrointest Surg. 2010 Feb;14 Suppl 1:S62-6. doi: 10.1007/s11605-009-1015-3. Epub 2009 Sep 23.
182. Fashner J, Gitu AC. Common gastrointestinal symptoms: risks of long-term proton pump inhibitor therapy. FP Essent. 2013 Oct;413:29-39. Review.
183. Saltzman JR1, Kowdley KV, Pedrosa MC, Sepe T, Golner B, Perrone G, Russell RM. Bacterial overgrowth without clinical malabsorption in elderly hypochlorhydric subjects. Gastroenterology. 1994 Mar;106(3):615-23.
184. Lombardo L1, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010 Jun;8(6):504-8. doi: 10.1016/j.cgh.2009.12.022. Epub 2010 Jan 6.
185. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013 May;11(5):483-490.
186. Bajaj JS1, Cox IJ2, Betrapally NS3, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol. 2014 Nov 15;307(10):G951-7. doi:10.1152/ ajpgi.00268. 2014. Epub 2014 Sep 25.
187. Untersmayr E, Scholl I, Schwoboda I, et al. Antacid medications inhibits digestion of dietary proteins and causes food allergy: A fish allergy model in Balb/c mice. J Allerg Clin Immun. 2003;112:616-623
188. Cohen S1, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophagal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol. 2015 Mar 5. doi: 10.1111/bcp.12619. [Epub ahead of print]
238. Kenneth E.L. McColl, Derek Gillen. Evidence That Proton-Pump Inhibitor Therapy Induces the Symptoms it is Used to Treat-EDITORIAL. Division of Cardiovascular & Medical Sciences, University of Glasgow, Gardiner Institute, Glasgow, UK. Gastroenterology, published online 01 June 2009. Refers to article: “Proton-Pump Inhibitor Therapy Induces Acid-Related Symptoms in Healthy Volunteers After Withdrawal of Therapy” 13 April 2009. Christina Reimer, Bo Sondergaard, Linda Hilsted, Peter Bytzer. Gastroenterology July 2009 (Vol. 137, Issue 1, Pages 80-87.e1)
239. Sonja Skrovanek, Katherine DiGuilio, et al. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol. 2014 November 15; 5(4): 496–513. Published online 2014 November 15.
240. Ueda K, Ueyama T, Oka M, Ito T, Tsuruo Y, Ichinose M. Polaprezinc (Zinc L-carnosine) is a potent inducer of anti-oxidative stress enzyme, heme oxygenase (HO)-1 – a new mechanism of gastric mucosal protection. J Pharmacol Sci. 2009 Jul;110(3):285-94. Epub 2009 Jun 19.