As we learned in the first part of this series, there are now more than 84,000 different chemicals that we are potentially exposed to on a daily basis and about 1,500 new chemicals are introduced each year.[1] The EPA estimates that in 2002 alone, over 4.7 billion pounds of toxic chemicals were released into the environment in the U.S.[2] We learned that only a quarter of more than 80,000 chemicals have been tested for toxicity.[3]
As we learned in the first part of this series, there are now more than 84,000 different chemicals that we are potentially exposed to on a daily basis and about 1,500 new chemicals are introduced each year.[1] The EPA estimates that in 2002 alone, over 4.7 billion pounds of toxic chemicals were released into the environment in the U.S.[2] We learned that only a quarter of more than 80,000 chemicals have been tested for toxicity.[3]
We also learned there are numerous chemicals we are exposed to on a daily basis in our homes and offices, in our cars and even in our bedrooms. Simply sitting at your desk at your home or office while reading this article on your computer exposes you to chemicals used in home and office construction, furniture, and electronics manufacturing.
Every time we eat food, drink tap water (or bottled water) or even breathe the air, we are being exposed to chemicals and potential toxins from the soil, food, air and water supplies. The totality of these chemicals in the human body is referred to as your chemical load.
Key concepts for this issue
- What is Your Toxic Load (Toxic Burden)?
- H
 ow Do We Defend Ourselves Against These Constant Threats?
ow Do We Defend Ourselves Against These Constant Threats? - Why Does Ability to Handle Toxins Vary From Person to Person?
- When Does Chemical Exposure Become a Problem?
- What is Toxicant-Induced Loss of Tolerance (TILT)?
- What Are the Consequences of Loss of Chemical Tolerance?
- Signs and Symptoms of Loss of Chemical Tolerance
- How Do We Lose Chemical Tolerance?
 What is Your Toxic Load?
What is Your Toxic Load?
How many chemicals are you exposed to on a daily basis? How many chemicals are in your food? As we found in the previous two segments, food is a common source of chemical exposures. Do you eat 100% organic food? Eating organic is always best whenever possible because it reduces chances of toxic exposure but doesn’t guarantee that your food is toxin-free. Why? Because there are toxins in the air, water and soil which make it into the food supply even though the food may be grown according to organic standards.
How about your drinking water? Do you drink bottled water or tap water? We learned that tap water can be contaminated with multiple chemicals, heavy metals, antibiotics and pharmaceuticals. Pharmaceutical residues and other organic wastewater contaminants have been shown to survive conventional water treatment processes and persist in water supplies.
A wide range of pharmaceuticals that include antibiotics, sex hormones, and drugs contaminate drinking water supplies of millions of Americans.[4]
“Environmental Working Group’s (EWG) studies show that tap water across the U.S. is contaminated with many industrial chemicals, and now we know that millions of Americans are also drinking low-level mixtures of pharmaceuticals with every glass of water.”[5]
How about the air we breathe? As we learned in the previous two segments, industrial sources of chemicals permeate the outdoor air we breathe and can lead to a wide variety of health effects, impacting not only our respiratory system but our immune system, endocrine system and nervous system. Industrial air pollution can have significant impacts on the brain and nervous system development. 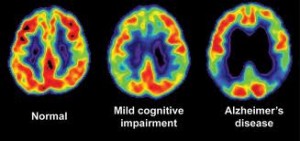 There have been brain changes associated with Alzheimer’s disease discovered in children exposed to outdoor air pollution. In a study published in 2012 in the prestigious Journal of Alzheimer’s Disease, brain changes normally associated with neurodegenerative diseases, such as Alzheimer’s disease, were observed in children exposed to air pollution:
There have been brain changes associated with Alzheimer’s disease discovered in children exposed to outdoor air pollution. In a study published in 2012 in the prestigious Journal of Alzheimer’s Disease, brain changes normally associated with neurodegenerative diseases, such as Alzheimer’s disease, were observed in children exposed to air pollution:
“40% of exposed children and young adults exhibit frontal tau hyperphosphorylation. 51% have beta-amyloid plaques compared to 0% in low pollution controls”[6]
 We also learned that our children are being exposed to hundreds of cancer-causing chemicals and toxins in umbilical cord blood before they are even born:
We also learned that our children are being exposed to hundreds of cancer-causing chemicals and toxins in umbilical cord blood before they are even born:
“Of the 287 chemicals we detected in umbilical cord blood, we know that 180 cause cancer in humans or animals, 217 are toxic to the brain and nervous system and 208 cause birth defects or abnormal development in animal tests”[7]
And even breast milk of American mothers has been found to be contaminated with toxic fire retardants:
“In the first nationwide tests for chemical fire retardants in the breast milk of American women, the EWG found unexpectedly high levels of these little-known neurotoxic chemicals in every participant tested”[8]
 It is impossible to protect yourself completely from all of these environmental chemicals and potential toxins. You will have exposures to hazardous chemicals during your lifetime and it will affect your health at various levels throughout your lifespan. The questions really are:
It is impossible to protect yourself completely from all of these environmental chemicals and potential toxins. You will have exposures to hazardous chemicals during your lifetime and it will affect your health at various levels throughout your lifespan. The questions really are:
- How much of a chemical load can you handle?
- How will you manifest symptoms of increased chemical load?
- How will this impact your health in the long term?
Let’s first take a look at how our bodies protect us from toxic exposures.
How Do We Defend Ourselves Against These Constant Threats?
The body’s ability to defend itself against such an arsenal of immune-producing triggers involves the integrity of our antioxidant systems, our hepatic biotransformation (liver detoxification) potential, the integrity of our barrier system (intestinal, pulmonary and blood-brain barrier) and most importantly our immune system’s ability to maintain chemical tolerance.
 The Bodies Antioxidant Systems
The Bodies Antioxidant Systems
The antioxidant systems of the body are critical for protecting the body from the harmful effects of various chemicals, toxins and heavy metals. These toxic compounds can produce excessive free radical activity leading to damage to important organelles, such as the ATP-producing mitochondria, and cells of various tissues of the body. Our body has the capacity to neutralize a certain amount of this free radical activity to prevent tissue damage. This relies on the antioxidant systems of the body, such as glutathione and superoxide dismutase, and the hepatic biotransformation pathways. However, once these antioxidant systems become overloaded with excessive oxidative stress, then oxidative damage can occur to our tissues and organs, leading to decreased organ function, chronic inflammation, and accelerated biological aging (see Successful Aging parts 1, 2).
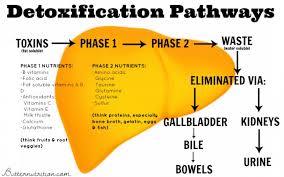 Hepatic Biotransformation (Liver Detoxification) Pathways
Hepatic Biotransformation (Liver Detoxification) Pathways
Hepatic biotransformation refers to the transformation of toxic lipid-soluble compounds and hormones into water-soluble compounds that can be excreted from the body via the urine. This is a process that involves multiple reactions. The first step is known as “phase I” and the second step is known as “phase II”. Phase I reactions involve the cytochrome P450 enzyme which is responsible for converting lipophilic (fat-loving) toxins into biotransformed intermediates that are more water-soluble. In phase II, these biotransformed intermediates undergo further biotransformation in a second series of enzymes called conjugases. These conjugases are enzymes that attach molecules, such as glucuronic acid, sulfate, glutathione, glycine, taurine or methyl groups to the biotransformed intermediates. These phase II pathways are named glucuronidation, sulfation, glutathione conjugation, glycine (amino acid) conjugation, acetylation and methylation.
 There are an enormous number of toxic compounds that can be handled and detoxified by the human biotransformation pathways. These toxins include not only environmental chemicals that we encounter in our food, air and water supply, medications, alcohol and drugs, but also endogenous compounds produced by the body, such as hormones and other signaling molecules. These endogenous compounds must be eliminated by the body as well, after they have served their purpose acting as signaling and messenger molecules. We now know that endogenously-produced hormones and other signaling molecules are not only detoxified by these enzymes, allowing for elimination, but also are metabolized into other signaling molecules allowing for more variation in the activities of these important substances. An example of this is estrogen metabolism.
There are an enormous number of toxic compounds that can be handled and detoxified by the human biotransformation pathways. These toxins include not only environmental chemicals that we encounter in our food, air and water supply, medications, alcohol and drugs, but also endogenous compounds produced by the body, such as hormones and other signaling molecules. These endogenous compounds must be eliminated by the body as well, after they have served their purpose acting as signaling and messenger molecules. We now know that endogenously-produced hormones and other signaling molecules are not only detoxified by these enzymes, allowing for elimination, but also are metabolized into other signaling molecules allowing for more variation in the activities of these important substances. An example of this is estrogen metabolism.
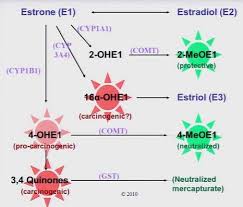 Estrogen, like all hormones, must be metabolized in the liver once it has served its purpose. There are several variations of these estrogen metabolites that result from metabolism of estrogen in these phase II pathways. Some of these metabolites have been associated with proliferation of tissues, such as seen in cancer, while other metabolites have been shown to be protective of these types of cancers. Main-taining a certain ratio of these metabolites is critical for health and imbalances of these ratios have been associated with breast cancer in women.[9]
Estrogen, like all hormones, must be metabolized in the liver once it has served its purpose. There are several variations of these estrogen metabolites that result from metabolism of estrogen in these phase II pathways. Some of these metabolites have been associated with proliferation of tissues, such as seen in cancer, while other metabolites have been shown to be protective of these types of cancers. Main-taining a certain ratio of these metabolites is critical for health and imbalances of these ratios have been associated with breast cancer in women.[9]
Having efficient and functional phase I and phase II hepatic biotransformation pathways is crucial to maintaining health. Studies have suggested an association between the ability of the body to adequately transform toxic xenobiotics and metabolites and the etiology of various chronic disease conditions, such as chronic fatigue syndrome (CFS), fibromyalgia (FMG) and multiple chemical sensitivities.[10] Research has also shown a connection between impairment of hepatic biotransformation and chronic neurodegenerative problems, such as Parkinson’s disease [11-13] and various cancers.[14-16]
 Hepatic Biotransformation Relies on Specific Nutrients
Hepatic Biotransformation Relies on Specific Nutrients
How do these enzyme systems work? What do they require to function? Like all metabolic pathways of the body, the enzyme systems involved in hepatic biotransformation require sufficient amounts of vitamins, minerals, coenzymes and cofactors in order to function. Specific detoxification pathways may be induced or inhibited depending on the presence of various available nutrients. For example, methylation conjugation relies on available methyl donors to function, such as vitamin B6, B12 and folic acid. Sulfation conjugation relies on the presence of the sulfur cofactor, which is obtained from precursors in the diet, such as methionine, cysteine and inorganic sulfur. Glutathione conjugation relies on available glutathione to take place, which in turn relies on various dietary cofactors and coenzymes. Besides available nutrients, there are many factors that influence these hepatic transformation pathways and one’s ability to handle toxic exposures, including gender, lifestyle habits, nutritional status, stress and genetics. As we will see, genetic differences in genes that regulate these detox pathways can make a big difference in one’s ability to handle toxic compounds.
 Why does ability to handle toxins vary from person to person?
Why does ability to handle toxins vary from person to person?
Why are most people living relatively normal, healthy lives despite all these toxic exposures while other people succumb to toxin-induced illness? The answer to these questions lies in genetic uniqueness of the genes involved in hepatic biotransformation. It is widely known that polymorphisms (genetic variations) exist throughout the human genome that can potentially affect any function related to the protein encoded by that particular gene. Moreover, the genes involved in these detoxification enzymes show a wide variety of polymorphisms which have been identified in the literature.[17] Polymorphisms in enzymes encoding phase I or phase II enzymes have been associated with increased prevalence and/or risk of many conditions including asthma, autism, cancers of the breast, prostate, lungs, esophagus, stomach and pancreas, multiple chemical sensitivity and cardiovascular disease.[18-24]
 Biochemical Individuality
Biochemical Individuality
The renowned biochemist, Roger Williams, Ph.D., was the first to use the term “biochemical individuality”. In his 1956 book of the same name, he described how the anatomical and physiological variations among people are related to their varied responses to the environment and to their differing nutritional needs. In 1950, Dr. Williams published an article in The Lancet entitled, “The Concept of Genetotrophic Disease”, in which he proposed a role for nutrients in the prevention of chronic disease.[25] Genetotrophic diseases are those for which genetic uniqueness creates a demand for specific nutrients beyond the average intake to facilitate optimal function and prevent premature disease. He theorized that when those specific needs are not met in a given individual, disease results. He further proposed that the major chronic degenerative diseases of aging are genetotrophic. In other words, the unique genes of each individual require different levels of nutrients for optimal health.  The consequences of not meeting these specific needs of the individual are expressed as degenerative disease “of unknown origin”. As our understanding of the interaction of genes and nutrition continues to increase, it appears that Dr. Williams’ theory may very well turn out to be the reality.
The consequences of not meeting these specific needs of the individual are expressed as degenerative disease “of unknown origin”. As our understanding of the interaction of genes and nutrition continues to increase, it appears that Dr. Williams’ theory may very well turn out to be the reality.
According to the work of Bruce Ames, Ph.D., biochemistry researcher who has studied the effect of nutrients on oxidative stress and DNA integrity, as many as one-third of all mutations in a gene result in the corresponding enzyme having a decreased affinity for its vitamin cofactor. This results in a lower reaction rate and requires increased amounts of vitamin cofactors in order for the enzyme reaction to take place at a higher rate. According to Dr. Ames, many of these effects can be ameliorated by giving the administration of high doses of the vitamin.[26]
When Does Chemical Exposure Become a Problem?
Over the years, hundreds of studies have explored the effects of environmental compounds on our health, turning up many disturbing findings. How much of an impact do these toxic compounds have on our health? A 2001 report suggested that between $568 billion and $793 billion is spent per year in the U.S. and Canada on environmentally-caused disease![27]
Environmental chemicals are linked with virtually every chronic health disorder including cancers, obesity, diabetes, heart disease, brain degeneration, neurological disorders and other health issues. But these links can be difficult for the average person to identify and our current healthcare system does not allow most doctors to spend much time searching for environmental contributions to their patients’ illness. Unless there is an acute exposure, chemical pollutants do not result in immediate symptoms and are often overlooked, but as we have seen, can have a wide variety of health effects over a period of time, affecting the nervous, immune and endocrine systems in multiple ways. Even small amounts of one toxic compound, such as mercury (still used in influenza vaccinations) can have multiple health effects:
 “Low concentrations of mercury chloride affect immune function in human cells by dysregulation of cytokine signaling with the potential to influence diverse health outcomes such as susceptibility to infectious disease or risk of autoimmunity”[28]
“Low concentrations of mercury chloride affect immune function in human cells by dysregulation of cytokine signaling with the potential to influence diverse health outcomes such as susceptibility to infectious disease or risk of autoimmunity”[28]
As we learned in part 1 of this series, the prevalence of allergenic-related diseases, food intolerance, and chemical sensitivities in both the pediatric and adult population has increased dramatically over the last two decades, with escalating rates of associated morbidity. A model of toxicity that explains this increase in these types of conditions that has been gaining attention in the literature is the model of toxicant-induced loss of tolerance (TILT).
 What is Toxicant-Induced Loss of Tolerance (TILT)?
What is Toxicant-Induced Loss of Tolerance (TILT)?
Normally, our body’s immune system is able to tolerate (remain non-reactive against) the various foods, chemicals and potential allergens that it comes in contact with on a daily basis. The TILT theory postulates that conditions of acquired allergy, food intolerance and chemical hypersensitivity are frequently the direct result of loss of chemical tolerance in response to a significant initiating toxic exposure. Here is how one researcher describes the development of loss of chemical tolerance and its relationship to the development of chronic disease:
“Following the primary toxicant insult, the individuals become sensitive to low levels of diverse and unrelated triggers in their environment, such as commonly encountered chemical, inhalant, or food antigens
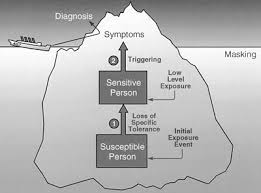 Among sensitized individuals, exposure to assorted inciting stimuli may precipitate diverse clinical and/or immune sequelae as may be evidenced by clinical symptoms as well as varied lymphocyte, antibody, or cytokine responses in some cases
Among sensitized individuals, exposure to assorted inciting stimuli may precipitate diverse clinical and/or immune sequelae as may be evidenced by clinical symptoms as well as varied lymphocyte, antibody, or cytokine responses in some cases
With escalating rates of toxicant exposure and bioaccumulation in the population-at-large, an increasing proportion of contemporary illness is the direct result of toxicant-induced loss of tolerance and ensuing sensitivity-related illness”[29]
 What Are the Consequences of Loss of Chemical Tolerance?
What Are the Consequences of Loss of Chemical Tolerance?
Chemical tolerance is our immune system’s ability to react proportionately to compounds such as toxins, pollutants and dietary proteins. There is no doubt certain toxins will lead to immediate loss of health and even death. However, many chronically ill individuals can no longer tolerate exposure to relatively benign exposures, such as perfume, detergents, jewelry, etc. These individuals have lost their chemical tolerance either from chemical/toxin exposure or from other mechanisms which can lead to loss of tolerance. For people with loss of chemical tolerance, trivial exposures can trigger a long list of conditions, including asthma, migraines, depression, fibromyalgia, fatigue, Gulf War syndrome, brain fog, memory loss, incontinence, neurological dysfunction, rashes, and so on.
 These people increasingly isolate themselves from the world and other people. They cannot tolerate many indoor places, other people’s scented body products, or clothes laundered in scented detergents. Even the smell of dryer sheets coming from a neighbor’s vent during a walk outside can make them sick. The loss of chemical tolerance is a phenomenon both researchers and clinicians have identified in more than a dozen countries and across diverse groups of people.
These people increasingly isolate themselves from the world and other people. They cannot tolerate many indoor places, other people’s scented body products, or clothes laundered in scented detergents. Even the smell of dryer sheets coming from a neighbor’s vent during a walk outside can make them sick. The loss of chemical tolerance is a phenomenon both researchers and clinicians have identified in more than a dozen countries and across diverse groups of people.
There was a great paper published on this theoretical model in 2001:
“In the late 1800’s, physicians observed that certain illnesses spread from sick, feverish individuals to those contacting them, paving the way for the germ theory of disease. The germ theory served as a crude, but elegant formulation that explained dozens of seemingly unrelated illnesses affecting literally every organ system. Today, we are witnessing another medical anomaly—a unique pattern of illness involving chemically exposed groups in more than a dozen countries, who subsequently report multisystem symptoms and new-onset chemical, food and drug intolerances.
These intolerances may be the hallmark of a new disease process or paradigm, just as a fever is a hallmark of infection. The fact that diverse demographic groups, sharing little in common except some initial chemical exposure event, develop these intolerances is a compelling anomaly pointing to a possible new theory of disease, one that has been referred to as “toxicant-induced loss of tolerance” (TILT).
 TILT has the potential to explain certain cases of asthma, migraine headaches, and depression, as well as chronic fatigue, fibromyalgia and Gulf War syndrome.”[30]
TILT has the potential to explain certain cases of asthma, migraine headaches, and depression, as well as chronic fatigue, fibromyalgia and Gulf War syndrome.”[30]
According to this theory, the issue is not how many toxins are in your system but whether your immune system reacts to them. This is the mechanism behind chemical intolerance, multiple chemical sensitivities, and toxin-induced brain degeneration mechanisms that are becoming so common today. In the literature, this is called “loss of chemical tolerance” or “toxic-induced loss of tolerance”. When people suffer from TILT, they are not necessarily sick from heavy metals and chemicals, rather, they are sick from their reaction to them.
Signs and Symptoms of Loss of Chemical Tolerance
- Asthma
- Skin rashes
- Weak immunity
- Fatigue
- Brain fog
- Depression
- Chronic pain
- Multiple chemical sensitivities
- Multiple food sensitivities
- Headaches/migraines
These are the people who cannot tolerate common chemicals without symptoms. Symptoms may vary but they are triggered by gas fumes, scented body products, laundry detergents, fabric softeners, new carpeting, new construction, the smell of new cars, and so on. For people with loss of chemical tolerance, trivial exposures can trigger a long list of conditions, including asthma, migraines, depression, fibromyalgia, fatigue, Gulf War syndrome, brain fog, memory loss, incontinence, neurological dysfunction, rashes, and so on.
These people increasingly isolate themselves from the world and other people. They cannot tolerate many indoor places, other people’s scented body products, or clothes laundered in scented detergents. Even the smell of dryer sheets coming from a neighbor’s vent during a walk outside can make them sick. It is common for them to feel increasingly angry at other people and understandably so. When a scented product triggers a migraine, incontinence or neurological symptoms, the person wearing it can seem cruel and selfish.
Summary of Symptoms of Loss of Chemical Tolerance
- Intolerance to smells
- Intolerance to jewelry
- Intolerance to shampoo, lotions, soaps and detergents, etc.
- Multiple food sensitivities
- Skin outbreaks
 How Do We Lose Chemical Tolerance?
How Do We Lose Chemical Tolerance?
Loss of chemical tolerance does not happen suddenly and for no reason. Chemical tolerance is dependent upon many overlapping physiological factors that include regulatory T-cell integrity, antioxidant reserves, barrier system integrity and integrity of hepatic biotransformation pathways. There are many ways you can lose self-tolerance or chemical tolerance and enter this vicious cycle:
- Heavy and ongoing chemical load can lead to depletion of glutathione and/or impaired
 hepatic biotransformation
hepatic biotransformation - If you have an ongoing chemical load that depletes glutathione, you will have impaired hepatic biotransformation, you will lose your ability to quench free radicals and oxidative stress, you will lose your ability to regulate T-cells and you will lose the integrity of your barrier systems
- As you lose your barrier systems, you will have increased inflammation and upregulation of NF-kB (an important inflammatory mediator) and increased expression of iNOS (very destructive to tissues) which creates more demand on glutathione sufficiency and increased loss of barrier systems
In summary, loss of chemical tolerance occurs when the following things take place:
- Depletion of glutathione
- Regulatory T-cell failure
- Breakdown of barrier systems
- Cytokine dysregulation/chronic inflammation
- Impaired hepatic biotransformation
 Depletion of Glutathione or Inefficient Glutathione System
Depletion of Glutathione or Inefficient Glutathione System
Depletion of glutathione is a primary cause of loss of chemical intolerance.[31] Glutathione is the body’s master antioxidant and the main antioxidant system in the body is the glutathione system. When there is oxidative stress (free radical activity) glutathione “takes the bullet” for our tissues and neutralizes potential damage from oxidative stress. Glutathione is also a very important regulator of immune responses because it influences regulatory T-cell function. In addition, glutathione is involved in both phase I and phase II hepatic biotransformation pathways and is your body’s primary method of elimination of heavy metals from the body, such as mercury and lead.[32]
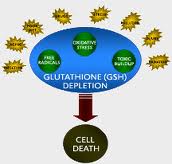 Excess exposure to environmental chemicals can be a problem but the research shows you do not have an immune response against environmental compounds until glutathione becomes depleted.[33] Even high levels of environmental compounds do not become an immune trigger unless the body’s glutathione levels are depleted. Acute toxic exposures (ie: mercury and other heavy metals) can deplete glutathione levels quickly and many multiple exposures over an extended period of time can also deplete glutathione stores.[34] If you live in a toxic environment, you will have more load to your antioxidant systems and will be at higher risk of glutathione depletion.
Excess exposure to environmental chemicals can be a problem but the research shows you do not have an immune response against environmental compounds until glutathione becomes depleted.[33] Even high levels of environmental compounds do not become an immune trigger unless the body’s glutathione levels are depleted. Acute toxic exposures (ie: mercury and other heavy metals) can deplete glutathione levels quickly and many multiple exposures over an extended period of time can also deplete glutathione stores.[34] If you live in a toxic environment, you will have more load to your antioxidant systems and will be at higher risk of glutathione depletion.
Here’s a summary of what happens in environmental exposures:
- Environmental exposures deplete glutathione
- Glutathione is an antioxidant and “takes the bullet” from the free radical
- If the glutathione system fails and you don’t have glutathione to take the bullet, then you get immune activation
- Immune activation then triggers inducible nitric oxide synthase (iNOS) which then causes tissue destruction of self-tissue[35]
 Regulatory T-cell failure
Regulatory T-cell failure
As discussed in previous segments, TH-3 cells, or regulatory T-cells, are a subpopulation of T-cells that downregulate the immune system, maintain tolerance to self-antigens and minimize autoimmune reactions. Regulatory T-cells are a component of the immune system that suppress immune responses from other cells. This is an important “self-check” mechanism inherent in the immune system to prevent excessive reactions. These cells are involved in shutting down the immune responses after they have successfully eliminated invading organisms and in the prevention of autoimmune responses and loss of chemical tolerance.[36] Glutathione depletion causes loss of regulatory T-cell function, as do vitamin D and omega-3 essential fatty acid deficiencies.[37-39] This can lead to dysregulation of immune responses and immune attacks against self-tissue (autoimmunity). Failure of the regulatory T-cells leads to overzealousness of certain aspects of the immune system and depressed in other aspects and has been associated with development of loss of chemical tolerance, multiple chemical sensitivity and autoimmunity.[40,41]
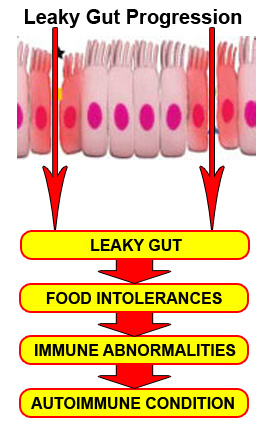 Breakdown of Barrier Systems
Breakdown of Barrier Systems
Glutathione depletion is also a major contributing factor to breakdown of the barrier systems, leading to leaky gut (intestinal permeability), compromised blood-brain barrier and breakdown of the pulmonary barrier in the lungs.[42] Many other dietary and lifestyle factors can break down these protective barriers of the body, increasing the risk for both loss of chemical intolerance and autoimmunity, including medications, stress, metabolic imbalances, dysbiosis or chronic inflammation.[43] Loss of one of these barrier systems leads to initiation of an inflammatory cas-cade which places greater demand on the glutathione system and risk of immune dysregulation and autoimmunity.[44]
NF-kB Activation and Chronic Inflammation
NF-kB activation and chronic inflammation is a major predisposing factor in loss of chemical tolerance. As we learned in earlier segments of this series, NF-kB controls the expression of genes encoding the proinflammatory mediators involved in chronic inflammation and autoimmune disease. NF-kB can become chronically elevated because of its own amplifying loop. [45] This means that elevations in NF-kB can perpetuate further elevations of NF-kB. NF-kB signaling needs to be downregulated in order to maintain tissue homeostasis. [46]
Common symptoms of chronic inflammation include:
- Bloating
- Skin rashes or eruptions
- Joint pain
- Brain fog
- Depression
- Anxiety
- Chronic pain
- Chronic fatigue
- Autoimmune flare-ups
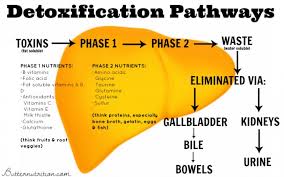 Impaired Hepatic Biotransformation
Impaired Hepatic Biotransformation
Environmental compounds and pollutants are metabolized into end-product metabolites by phase I and phase II reactions that can then be excreted by urine, sweat or feces. If hepatic biotransformation pathways are compromised, the pollutants may not be able to be metabolized or are metabolized into more immunoreactive end-products that both increase oxidative stress and further promote the vicious cycle of impaired chemical tolerance. If liver function becomes compromised, it can increase the toxic load and further impair chemical tolerance. Likewise, toxic exposures and the associated inflammation can then hinder liver metabolic function.
Summary of mechanisms that may be involved in loss of chemical tolerance:
 Heavy and ongoing chemical load can lead to depletion of glutathione and/or impaired hepatic biotransformation
Heavy and ongoing chemical load can lead to depletion of glutathione and/or impaired hepatic biotransformation- Depletion of glutathione and impaired hepatic biotransformation, leads to loss of ability to quench free radicals and increased oxidative damage, inability to regulate T-cells and loss of integrity of the barrier systems
- Loss of barrier systems leads to increased inflammation, upregulation of NF-kB and increased expression of iNOS which creates more demand on glutathione sufficiency and increased loss of barrier systems
In the next segment of this series, we will cover the following topics:
- The Role Chemical Sensitivity Plays in the Development of Autoimmunity
- Does Chemical Tolerance Vary From Person to Person?
- Laboratory Evaluation of Immune-Chemical Tolerance
- Other Factors Besides Chemical Exposure That Impact Chemical Sensitivity
- Nutritional Management of Loss of Chemical Tolerance
- Heavy Metal Exposure and Elimination
References
- Moreno MA, Furtner F, Rivara FP. Children’s environmental health. Arch Pediatr Adolesc Med. 2012 Oct;166(10):976. doi: 10.1001/2013.jamapediatrics.8.
2. http://www.epa.gov/All+chemicals&industry
3. The legal failure to prevent subclinical developmental toxicity. Basic Clin Pharmacol Toxicol. 2008 Feb;102(2):267-273
4. http://usatoday30.usatoday.com/news/nation/2008-03-10-drugs-tap-water_N.htm?loc
5. http://www.ewg.org/news/testimony-official-correspondence/pharmaceuticals-pollute-us-tap-water
6. White matter hyperintensities, systemic inflammation, brain growth and cognitive functions in children exposed to air pollution. J Alzheimer’s Dis. 2012;31(1):183-191
7. Houlihan J, et al. Body burden: the pollution in newborns. July 14, 2005. Environmen-tal Working Group.http//www.ewg.org/research/body-burden-pollution-newborns
8. Lunder S, Sharp R. Toxic fire retardants in human breast milk. Sept 23, 2003. Environmental Working Group. http//www.ewg.org/research/mothers-milk-0
9. Santen RJ, Yue W, Wang JP. Estrogen metabolites and breast cancer. Steroids. 2014 Aug 26. pii: S0039-128X(14)00196-2. doi: 10.1016/j.steroids.2014.08.003. [Epub ahead of print] Review.
10. Bland J, Barrager E, Reedy R, Bland K. A medical food-supplemented detoxification program in the management of chronic health problmes. Altern Ther Health Med. 1995 Nov 1;1(5):62-71.
11. Steventon G, Heafield M, Waring R, Williams A. Xenobiotic metabolism in Parkinson’s disease. Neurology. 1989 ;39:883-887.
12. Huang Y, et al. Genetic contributions to Parkinson’s disease. Brain Res Brain Res Rev. 2004;46(1):44-70.
13. Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003; 302(5646):819-22.
14. Ning B, Wang C, Morel F, et al. Human glutathione S-transferase A2 polymorphisms: variant expression, distribution in prostate cancer cases/controls and a novel form. Pharmacogenetics. 2004;14(1):35-44.
15. Mohrenweiser HW. Genetic variation and exposure related risk estimate: will toxicology enter a new era: DNA repair and cancer as a paradigm. Toxicol Pahtol. 2004;32(Suppl 1):136-45.
16. Chacko P, Joseph T, Mathew BS, et al. Role of xenobiotic metabolizing gene polymorphisms in breast cancer susceptibility and treatment outcome. Mutat Res. 2005;581(1-2):153-63.
17. Textbook of Functional Medicine. Institute of Functional Medicine. 2005.
18. Tamer L, Calikoglu M, Ates NA, et al. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirologoy. 2004;9(4):493-498.
19. Sazci A, Ergul E, Utkan NZ, et al. Catechol-O-methyltransferase Val 108/158 Met polymorphism in premenopausal breast cancer patients. Toxicology. 2004;204(2-3):197-202.
20. Nakazato H, Suzuki K, Matsui H, et al. Association of genetic polymorphisms of glutathione-S-transferase genes (GSTM1, GSTT1, GSTP1) with familial prostate cancer risk in a Japanese population. Anticancer Res. 2003;23(3C):2897-2902.
21. Shi WX, Chen SQ. Frequencies of poor metabolizers of cytochrome P450 2C19 in esophagus cancer, stomach cancer, lung cancer and bladder cancer in Chinese population. Worl J Gastroenterol. 2004;10(13):1961-63
22. Ockenga J, Vogel A, Teich N, et al. UDP glucuronosyltransferase (UGT1A7) gene polymorphisms increase th risk of chronic pancratitis and pancreatic cancer. Gastroenterology. 2003;124(7):1802-8.
23. McKeown-Eyssen G, Baines C, Cole DE, et al. Case-control study of genotypes in multiple chemical sensitivity: C YP2D6, NAT1, NAT2, PON1, PON2 and MTHFR. Int J Epidemiol. 2004;33(5):971-978.
24. Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomic of the paraoxonase (PON1) polymporphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371-392.
25. Williams RJ, Beerstecher E Jr, Berry LJ. The concept of genetotrophic disease. Lancet. 1950;1(7):287-89
26. Textbook of Functional Medicine. Institute of Functional Medicine. 2005.
27. Muir T, Zegar M. Societal costs of exposure to toxic substances: economic and health costs of four case studies that are candidates for envrironmental causation. Environ Health Perspect. 2001;109 Suppl 6:885-903.
28. Gardner RM, Nyland JF, Evans SL, et al. Mercury induces an unopposed inflammatory resonse in human peripheral blood mononuclear cells in vitro. Environ Health Perspect. 2009 Dec;117(12):1932-38
29. Sensitivity-related illness: the escalating pandemic of allergy, food intolerances and chemical sensitivity. Sci Total Environ. 2010 Nov 15; 408(24):6047-6061.
30. The compelling anomaly of chemical intolerance. Ann NY Acad Sci. 2001 Mar; 933-23
31. Requejo R, Tena M. Influence of glutathione chemical effectors in the response of maize to arsenic exposure. J Plant Physiol. 2012 May 1;169(7):649-656
32. Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, Maes M. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. 2014 Dec;50(3):1059-84. doi: 10.1007/s12035-014-8705-x. Epub 2014 Apr 22. PMID: 24752591
33. Critical role of hydrogen peroxide in the differential susceptibility of TH1 and TH2 cells to tributyltin-induced apoptosis. Biochem Pharmacol. 2008 Jan 15;75(2):552-61. Epub 2007 Sept 18
34. Carocci A, Rovito N, Sinicropi MS, Genchi G. Mercury toxicity and neurodegenerative effects. Rev Environ Contam Toxicol. 2014;229:1-18. doi: 10.1007/978-3-319-03777-6_1. Review.
35. GSH-dependent iNOS and HO-1 mediated apoptosis of human Jurkat cells induced by nickel (II). Environ Toxicol. 2009 Aug; 24(4):404-14
36. Micovic V, Vojnikovic B, Bulog A, Coklo M, Mlatestinic D, Mrakovcic-Sutic I. Regulatory T cells (Tregs) monitoring in environmental diseases. Coll Antropol. 2009 Sep;33(3):743-746
37. Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3071-6.
38. Mocanu V, Oboroceanu T, Zugun-Eloae F. Current status in vitamin D and regulatory T cells–immunological implications. Rev Med Chir Soc Med Nat Iasi. 2013 Oct-Dec;117(4):965-73.
39. Issazadeh-Navikas S, Teimer R, Bockermann R. Influence of dietary components on regulatory T cells.
Mol Med. 2012 Feb 10;18:95-110. doi: 10.2119/molmed.2011.00311. Review.
40. Winder C. Mechanisms of multiple chemical sensitivity. Toxicol Lett. 2002Mar 10;128(1-3):85-97
41. Alla Skapenko, Jan Leipe, Peter E Lipsky, Hendrik Schulze-Koops. The role of the T cell in autoimmune inflammation. Arthritis Res Ther. 2005; 7(Suppl 2): S4–S14.
42. Intestinal barrier function in response to abundant or depleted mucosal glutathione in Salmonella-infected rats. BMC Physiol. 2009 Apr 17:9:6
43. Stephan C Bischoff, Giovanni Barbara, Wim Buurman, Theo Ockhuizen, Jörg-Dieter Schulzke, Matteo Serino, Herbert Tilg, Alastair Watson, Jerry M Wells. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014; 14(1): 189. Published online 2014 November 18. doi: 10.1186/s12876-014-0189-7
44. Stephan C Bischoff, Giovanni Barbara, Wim Buurman, Theo Ockhuizen, Jörg-Dieter Schulzke, Matteo Serino, Herbert Tilg, Alastair Watson, Jerry M Wells. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014; 14(1): 189. Published online 2014 November 18. doi: 10.1186/s12876-014-0189-7
45. Nuclear factor-kB—a pivotal transcription factor in chronic inflammatory diseases. NF-kB Inflammatory Amplifying Loop. N Eng J Med. 1997;336:1066-1071.
46. Return to homeostasis: downregulation of NF-kB responses. Nat Immunol. 2011 Jun 19;12(8):709-714