 In this issue we look at the prevalence of autoimmune disease, the stages and characteristics of autoimmune disease and discuss the major areas of immune dysfunction involved in the progression of these diseases.
In this issue we look at the prevalence of autoimmune disease, the stages and characteristics of autoimmune disease and discuss the major areas of immune dysfunction involved in the progression of these diseases.
 Autoimmune Disease and the Major Areas of Immune Dysfunction in Autoimmunity
Autoimmune Disease and the Major Areas of Immune Dysfunction in Autoimmunity
Key points of this issue:
- The prevalence of autoimmune disease is increasing every year
- 1 out of 12 women and 1 out of 24 men have an autoimmune disease
- Autoimmunity results when the body’s immune system loses tolerance to the body’s self-tissue and begins targeting self-tissue for destruction
- The three stages of autoimmune disease
- Autoimmune disease is an incurable condition
- Characteristics of an autoimmune condition
- The main mechanisms of immune dysfunction in autoimmunity
The Autoimmune Epidemic
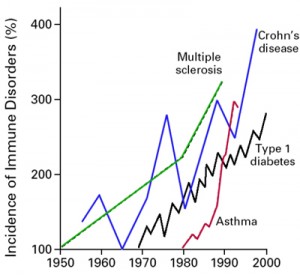
There is an explosion of autoimmune disease taking place worldwide. According to the American Autoimmune Related Disease Association’s current statistics, “Approximately 50 million Americans, 20 percent of the population or one in five people, suffer from autoimmune diseases.” (1) The National Institutes of Health estimates up to 23.5 million Americans have an autoimmune disease. In comparison, cancer affects up to 9 million and heart disease up to 22 million. These numbers are staggering because they are referring to disease already taking place, not early-stage conditions that lead to autoimmune disease. They also point out that medical education provides minimal learning about autoimmune disease and that specialists are generally unaware of interrelationships among the different autoimmune diseases or advances in treatment outside their own specialty area. (2)
 Autoimmune Disease in the U.S.
Autoimmune Disease in the U.S.
- Increasing every year
- Most cases are not diagnosed
- Most cases continue to progress
- Conventional healthcare has very little to offer besides steroids and immune-suppressing medications
- Conventional and alternative healthcare may not fully understand autoimmunity or even immunology
Consider the Following Autoimmune Statistics and Facts (www.aarda.org):
- “The National Institutes of Health estimates up to 23.5 million Americans suffer from autoimmune disease and that the prevalence is rising”
- “1 out of 12 women and 1 out of 24 men have an autoimmune disease”
- “Researchers have identified 80-100 different autoimmune diseases and suspect at least 40 additional diseases of having an autoimmune basis. These diseases are chronic and can be life-threatening”
- “Autoimmune disease is one of the top 10 leading causes of death in female children and women in all age groups up to 64 years of age”
- “A close genetic relationship exists among autoimmune disease, explaining clustering in individuals and families as well as a common pathway of disease”
- “Commonly used immunosuppressant treatments lead to devastating long-term side-effects”
- “The Institute of Medicine reports that the U.S. is behind other countries in research into immune system self-recognition, the process involved in autoimmune disease”

- “Symptoms cross many specialties and can affect all body organs”
- “Medical education provides minimal learning about autoimmune disease”
- “Specialists are generally unaware of interrelationships among the different autoimmune diseases or advances in treatment outside their own specialty area”
- “Initial symptoms are often intermittent and unspecific until the disease becomes acute”
- “Research is generally disease-specific and limited in scope. More information-sharing and crossover among research projects on different autoimmune diseases is needed”
- “The NIH estimates up to 23.5 million Americans have an autoimmune disease. In comparison, cancer affects up to 9 million and heart disease up to 22 million”
- “According to researchers from the Mayo Clinic, one in 12 women and one in 20 men will develop an autoimmune disease”
 What is Autoimmune Disease?
What is Autoimmune Disease?
Autoimmune (AI) disease results when the immune system begins targeting self-tissue of one’s own body causing destruction of that tissue or organ. One of the roles of the immune system is to distinguish between “self” and “non-self”. This means that a healthy immune system can determine when a foreign pathogen, such as a bacteria or virus, enters the body and initiates an immune response against that foreign pathogen to eliminate or destroy it. It does not normally attack the cells or tissue of one’s own body. Normally, self-tissue is “tolerated” by one’s own immune system. However, in some individuals, this tolerance to self-tissue is lost. This is exactly what happens in autoimmune disease. The tissue that gets targeted and destroyed by the immune response defines the type of autoimmune disease one develops. However, all AI disease involves similar mechanisms of immune dysregulation so these seemingly different diseases can be treated similarly by modulating some aspect of the immune response.
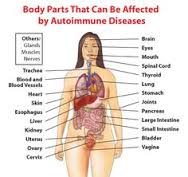 There are many different AI diseases that have been identified, such as rheumatoid arthritis (RA), multiple sclerosis (MS), lupus (SLE), Hashimoto’s thyroiditis, Crohn’s disease, ulcerative colitis (UC), type I diabetes and Celiac disease (CD). This is because AI disease can affect every organ and tissue in the body.
There are many different AI diseases that have been identified, such as rheumatoid arthritis (RA), multiple sclerosis (MS), lupus (SLE), Hashimoto’s thyroiditis, Crohn’s disease, ulcerative colitis (UC), type I diabetes and Celiac disease (CD). This is because AI disease can affect every organ and tissue in the body.
“Autoimmune diseases can affect virtually every site in the body”
“At least 15 diseases are known to be the direct result of an autoimmune response, and circumstantial evidence links more than 80 conditions to autoimmunity.”(3)
 How Does Autoimmunity Happen?
How Does Autoimmunity Happen?
Autoimmunity results when the body’s immune system loses tolerance to the body’s self-tissue and begins targeting self-tissue for destruction. The details of precisely how this self-tolerance is lost in susceptible individuals is not yet clearly understood. But there is growing evidence that a variety of factors may play a role in loss of self-tolerance, including genetics, environmental pollutants and toxins, hormonal changes, immunoreactive dietary proteins (from our food), leaky gut, chronic infections, chronic inflammation and stress responses. (For a more detailed explanation of the mediators and modulating factors leading to autoimmune disease, click here.) All of these factors lead to immune dysregulation which leads to loss of self-tolerance and the production of antibodies against self-tissue. These autoantibodies indicate that that specific tissue of the body is being targeted for destruction by the immune system and serve as the hallmark of autoimmune processes in the body and aid in diagnosis of autoimmune disease.
“The origins of autoimmune disease are multifactorial. Environmental factors and a genetic predisposition result in tissue injury caused by autoreactive T cells or antibodies.”(4)
 “Emerging evidence has suggested the involvement of environmental factors such as infections and xenobiotics, and some dietary proteins and their antibodies in the pathogenesis of many autoimmune diseases”(5)
“Emerging evidence has suggested the involvement of environmental factors such as infections and xenobiotics, and some dietary proteins and their antibodies in the pathogenesis of many autoimmune diseases”(5)
“The cause may be due to environmental factors such as bacterial or viral infections, or haptenic toxic chemicals binding to human tissue, causing modification of self-antigens and the subsequent production of autoantibodies”.(6)
The Stages of Autoimmunity
 It is important to keep in mind that the development of autoimmune disease does not occur overnight. The manifestations of the disease occur much later than the initiating factors that lead to the development of autoimmunity. It may take years for the targeting of self tissue by autoantibodies to result in autoimmune disease. During this time, patients are often asymptomatic and unaware that they are laying the groundwork for the development of autoimmune disease.
It is important to keep in mind that the development of autoimmune disease does not occur overnight. The manifestations of the disease occur much later than the initiating factors that lead to the development of autoimmunity. It may take years for the targeting of self tissue by autoantibodies to result in autoimmune disease. During this time, patients are often asymptomatic and unaware that they are laying the groundwork for the development of autoimmune disease.
“Autoimmune diseases tend to have long, asymptomatic prodromal periods and the initiating events leading to loss of self tolerance occur long before the disease becomes clinically manifest.”(7)
 Autoimmunity in general, takes place in several stages:
Autoimmunity in general, takes place in several stages:
The first stage is called silent autoimmunity and is characterized by the onset of loss of immune self-tolerance without any loss of self-tissue. This stage is identified by elevated autoantibodies but without any other signs or symptoms.
The second stage is called autoimmune reactivity and is the stage when the autoimmunity has progressed into tissue destruction and is starting to create noticeable symptoms associated with loss of function but no severe destruction of the tissue.
The third stage is called autoimmune disease and this is when there is clear and significant loss of self-tissue that can be identified with imaging studies and other tests, such as nerve conduction studies.(8)
Autoimmune Disease is an Incurable Condition
 There is no known cure for autoimmune disease. The expression of the disease can go into remission but that does not mean the person is cured. Unfortunately, some healthcare providers and patients confuse autoimmune remission with cure.
There is no known cure for autoimmune disease. The expression of the disease can go into remission but that does not mean the person is cured. Unfortunately, some healthcare providers and patients confuse autoimmune remission with cure.
It is clear that once the gene expression of autoimmune disease turns on, there is no “off” switch, but there is a “dimmer switch” that can modulate how active the expression becomes.
With the explosion of autoimmunity taking place worldwide, many chronic patients who seek help are either not properly diagnosed or are overlooked and eventually seek the care of alternative medicine practitioners for help. Unfortunately, most alternative healthcare providers are not adequately trained to identify, monitor and manage autoimmune-related conditions. Most autoimmune disease remains undiagnosed. However a healthy diet and lifestyle provide many autoimmune patients with greater function and overall health while remaining undiagnosed.
 Characteristics of an Autoimmune Condition
Characteristics of an Autoimmune Condition
- Incurable condition
- Relapsing and remitting
- Chronic and progressive
- Diagnosed with conditions associated with autoimmunity
- Hypothyroidism
- Diabetes
- Idiopathic neuropathy
- Inflammatory bowel disease
- Meniere’s disease
- Arthritis (systemic)
Mechanisms of Autoimmunity
There are several mechanisms of immune dysfunction which have been shown to play a role in the development of autoimmune reactivity and autoimmunity. We will discuss each of these mechanisms briefly and show how they play a role in autoimmunity according to the most recent literature in the field of immunology. This section is a bit technical, so if you are not the technical type, then just skip down to the next section. In the next article, we will show how each of these mechanisms are can be modulated with natural compounds in the management of these conditions under the supervision of a functional medicine practitioner.
 Major Areas of Immune Dysfunction in AI Disease
Major Areas of Immune Dysfunction in AI Disease
- NF-kB activation and dampening
- Glutathione recycling system
- Barrier system integrity
- TH-1 and TH-2 polar shifts
- TH-3 or regulatory T-cell activation
- TH-17 activation and dampening
- Nitric oxide isomer expressions
 NF-kB Activation and Dampening
NF-kB Activation and Dampening
An increasing number of studies indicate that a protein complex known as NF-kB plays an important role in controlling the expression of genes related to the development of autoimmunity. (9) NF-kB controls the expression of genes encoding the proinflammatory mediators (ie: cytokines, chemokines, and adhesion molecules) involved in autoimmune disease.
NF-kB can become chronically elevated because of its own amplifying loop. (10) This means that elevations in NF-kB can perpetuate further elevations of NF-kB. Some activation of NF-kB is essential for a healthy immune response. However, after the pathogen is eliminated, NF-kB signaling needs to be downregulated to maintain tissue homeostasis. (11) If this does not occur, then immune dysregulation takes place leading to autoimmunity. Recent data has shown that NF-kB is required for activation of immune cells against self-tissue, and its hyperactivity should be minimized, since it promotes autoimmunity. (12)
 The Glutathione Recycling System
The Glutathione Recycling System
Glutathione is a tripeptide compound that acts as an immune modulator, antioxidant, and hepatic detoxification substrate. Glutathione is the main protecting antioxidant for cellular mitochondria. Glutathione exists in reduced (GSH) and oxidized (GSSG) states. GSH (reduced glutathione) becomes GSSG (oxidized glutathione) by glutathione peroxidase to support anti-inflammatory response mechanisms. GSH can be regenerated from GSSG by the enzyme glutathione reductase to keep the cycle going. The ability to recycle GSH is critical for a healthy immune response. As long as the enzymes glutathione peroxidase and glutathione reductase are working efficiently, your body is able to maintain this glutathione recycling system and you are fine. When this system fails, you get immune dysregulation and increased risk of autoimmunity.
 Glutathione appears to be an important antioxidant that helps support regulatory TH-3 cells of the immune system (important immune-regulating cells), protect the intestinal barrier and quench oxidative compounds before they are activated by the NF-kB receptor. Many studies suggest that intracellular GSH levels in antigen-presenting cells such as macrophages, influence the TH1/TH2 cytokine response patterns which are associated with immune dysregulation and autoimmunity. The observations reported in one study showed that pro-GSH molecules represent new therapeutic agents to support immune modulation. (13) Another study suggested that the severity of some autoimmune diseases, such as systemic lupus erythmatosus (SLE), correlate with levels of glutathione in the blood, namely, the lower the glutathione levels, the more severe the autoimmune disease. (14)
Glutathione appears to be an important antioxidant that helps support regulatory TH-3 cells of the immune system (important immune-regulating cells), protect the intestinal barrier and quench oxidative compounds before they are activated by the NF-kB receptor. Many studies suggest that intracellular GSH levels in antigen-presenting cells such as macrophages, influence the TH1/TH2 cytokine response patterns which are associated with immune dysregulation and autoimmunity. The observations reported in one study showed that pro-GSH molecules represent new therapeutic agents to support immune modulation. (13) Another study suggested that the severity of some autoimmune diseases, such as systemic lupus erythmatosus (SLE), correlate with levels of glutathione in the blood, namely, the lower the glutathione levels, the more severe the autoimmune disease. (14)
Glutathione is also suggested to play an important role in gut barrier function and prevention of intestinal inflammation. One study showed that intestinal barrier destruction occurs once glutathione is depleted. It also showed that glutathione depletion aggravated intestinal inflammation after infection. (15) In another study, the researchers concluded that their data support the hypothesis that the GSH antioxidant system plays a crucial role in gut barrier protection. (16)
Intestinal Barrier System Integrity
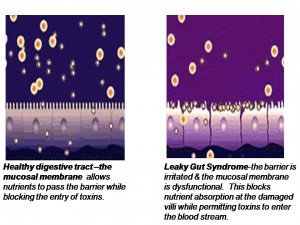 When the intestinal mucosa of the digestive tract is inflamed, the tight junctions of the intestinal mucosa are compromised and they become widened and permeable to large, undigested compounds, toxins and bacteria which can then enter the bloodstream. This condition is known as leaky gut. (What is Leaky Gut?) These large undigested proteins that are absorbed are reacted against by the underlying intestinal immune system and promote exaggerated immune responsiveness (inflammation) which leads to further destruction of the intestinal barrier. This creates a vicious cycle of further intestinal inflammation and greater loss of intestinal integrity. As the intestinal tract becomes inflamed from diet, lifestyle, medications, and infections, it promotes further intestinal inflammation and further intestinal permeability.
When the intestinal mucosa of the digestive tract is inflamed, the tight junctions of the intestinal mucosa are compromised and they become widened and permeable to large, undigested compounds, toxins and bacteria which can then enter the bloodstream. This condition is known as leaky gut. (What is Leaky Gut?) These large undigested proteins that are absorbed are reacted against by the underlying intestinal immune system and promote exaggerated immune responsiveness (inflammation) which leads to further destruction of the intestinal barrier. This creates a vicious cycle of further intestinal inflammation and greater loss of intestinal integrity. As the intestinal tract becomes inflamed from diet, lifestyle, medications, and infections, it promotes further intestinal inflammation and further intestinal permeability.
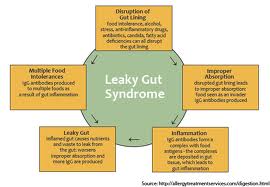 It is not uncommon for individuals to suffer from ongoing leaky gut syndrome for several years. After the intestinal mucosa becomes damaged, the damaged cells become unable to properly absorb nutrients and produce the enzymes necessary for digestion and absorption. This leads to malnutrition, further intestinal inflammation, further intestinal permeability and the development of food sensitivities, bacterial overgrowths, yeast overgrowths and impaired intestinal immune integrity. These self-promoting vicious cycles become difficult to unwind unless aggressive dietary and nutritional strategies are employed.
It is not uncommon for individuals to suffer from ongoing leaky gut syndrome for several years. After the intestinal mucosa becomes damaged, the damaged cells become unable to properly absorb nutrients and produce the enzymes necessary for digestion and absorption. This leads to malnutrition, further intestinal inflammation, further intestinal permeability and the development of food sensitivities, bacterial overgrowths, yeast overgrowths and impaired intestinal immune integrity. These self-promoting vicious cycles become difficult to unwind unless aggressive dietary and nutritional strategies are employed.
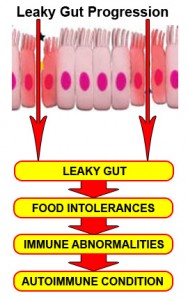 Most importantly, the immune dysregulation from increased permeability has been shown to perpetuate immune overzealousness and loss of ability to regulate self and nonself tolerance, leading to autoimmunity.
Most importantly, the immune dysregulation from increased permeability has been shown to perpetuate immune overzealousness and loss of ability to regulate self and nonself tolerance, leading to autoimmunity.
“When the finely-tuned trafficking of macromolecules is dysregulated in genetically susceptible individuals, both intestinal and extraintestinal autoimmune disorders can occur.”(17)
This review paper was published in 2009 in the Annals of N.Y. Academy of Science, one of the top-rated most powerful scientific journals available:
“There is growing evidence that increased intestinal permeability plays a pathogenic role in various autoimmune diseases. Therefore, we hypothesize that loss of intestinal barrier function is necessary to develop autoimmunity.”(18)
 TH-1 and TH-2 Polar Shifts
TH-1 and TH-2 Polar Shifts
The immune system has two major immune responses:
- T-helper-1 (TH-1) response, which is mediated by T-cells
- T-helper-2 (TH-2) response, which is mediated by B-cells
Both immune responses are important for protecting the body against foreign microbes and invaders. In autoimmunity, there is a polar shift or dominance of either the TH-1 or TH-2 system. Many autoimmune diseases are classified in the immunology literature by their T-helper dominance. The shift in their TH-1 or TH-2 systems remains constant and does not change over the course of the autoimmunity. Identification of the shift may help an individual identify which natural compounds activate their autoimmunity and which natural compounds help potentially dampen their autoimmunity. This identification is helpful in modulating the immune responses seen in autoimmunity.
 TH-3 Cells or Regulatory T-Cell Activation
TH-3 Cells or Regulatory T-Cell Activation
TH-3 cells, or regulatory T-cells, are a subpopulation of T-cells that downregulate the immune system, maintain tolerance to self-antigens and minimize autoimmune disease. Regulatory T-cells are a component of the immune system that suppress immune responses from other cells. This is an important “self-check” mechanism inherent in the immune system to prevent excessive reactions. These cells are involved in shutting down the immune responses after they have successfully eliminated invading organisms and also in preventing autoimmune TH-1 and TH-2 polar shifts.
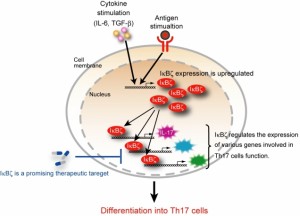 TH-17 Activation and Dampening
TH-17 Activation and Dampening
T-helper 17 (TH-17) is a subset of T-helper cells producing IL-17. IL-17 is a potent proinflammatory cytokine that amplifies ongoing inflammation by inducing the expression of tumor necrosis factor-α (TNF- α), IL-1β, and IL-6 in epithelial and endothelial cells. TH-17 cells serve a very important function in anti-microbial immunity and are important for immunity and protection from opportunistic infections. Therefore, TH-17 is important for normal immunity, but it can also become excessively active, especially in those with autoimmunity. These cells are considered developmentally distinct from TH-1 and TH-2 cells, and excessive amounts of these cells are thought to play a key role in autoimmune disease activation. TH-17 activity is considered a key marker in the activation and severity of autoimmune flare-ups.
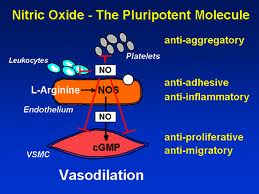 Nitric Oxide Isomer Expressions
Nitric Oxide Isomer Expressions
Nitic oxide (NO) is an important messenger molecule involved in many physiological and pathological processes as well as a chemical-signalling molecule that is involved with communication in the nervous system, immune system and vascular system. It was proclaimed the “Molecule of the Year” in 1992. (19)
Nitic oxide plays both destructive and protective roles in autoimmunity, depending on which of the three enzymes, or synthases, is expressed. The three nitric oxide synthase (NOS) isomers (forms) are neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS). nNOS supports nervous tissue, including the brain, and neurological synapses involved in neuronal signaling and brain function. eNOS activity supports blood vessel health by producing dilation of blood vessels, dissolves endothelial plaques, and enhances blood flow. iNOS, however, has destructive effects on tissue. iNOS is activated by TH-17 and produces harmful free radical action and initiates tissue destruction. How these isomers are manipulated or modulated will determine how much destruction of tissue or ability to recover from autoimmune flare-ups a person has.
Studies show that nitric oxide (NO) modulates inflammatory and immune responses in multiple ways and plays a protective role in many forms of inflammatory and autoimmune reactions. (20, 21, 22, 23)
“We conclude that NO plays a critical immunoregulatory role and NO modulation may prevent the onset of autoimmune reactions”(24)
The Primary Difference Between Conventional vs. Functional Medicine in Management of Autoimmune Conditions
 The primary medications still being used by conventional medicine to manage autoimmune disease are immune-suppressing medications. Up until recently, the primary class of drugs used to manage most autoimmune disease was oral corticosteroids, such as prednisone, which have powerful anti-inflammatory effects. However, there are many known side-effects of these medications, especially when taken long-term. Some of the known side effects of steroids include immune suppression, bone loss, neurodegenerative impacts on the brain, insulin-related issues and impacts on blood sugar regulation, epithelial thinning, and catabolic states which means these patients have difficulty recovering from flare-ups from their autoimmunity.
The primary medications still being used by conventional medicine to manage autoimmune disease are immune-suppressing medications. Up until recently, the primary class of drugs used to manage most autoimmune disease was oral corticosteroids, such as prednisone, which have powerful anti-inflammatory effects. However, there are many known side-effects of these medications, especially when taken long-term. Some of the known side effects of steroids include immune suppression, bone loss, neurodegenerative impacts on the brain, insulin-related issues and impacts on blood sugar regulation, epithelial thinning, and catabolic states which means these patients have difficulty recovering from flare-ups from their autoimmunity.
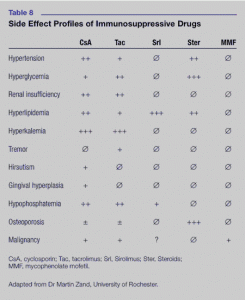 “Osteoporosis, adrenal suppression, hyperglycemia, dyslipidemia, cardiovascular disease, Cushing’s syndrome, psychiatric disturbances and immunosuppression are among the more serious side effects noted with systemic corticosteroid therapy, particularly when used at high doses for prolonged periods.”(25)
“Osteoporosis, adrenal suppression, hyperglycemia, dyslipidemia, cardiovascular disease, Cushing’s syndrome, psychiatric disturbances and immunosuppression are among the more serious side effects noted with systemic corticosteroid therapy, particularly when used at high doses for prolonged periods.”(25)
The newer class of medications called “biologic therapies” which are more specific in their therapeutic targets of the immune system than previous medications, such as disease-modifying anti-rheumatic drugs (DMARDs) and corticosteroids, still have serious immune-suppressive effects. This is why people are still at risk of developing various types of infection such as tubercolosis. Another major risk of these medications is the induction of autoimmune disease (marked by anti-DNA antibodies) in up to 1/3 of users. (26) They are also extremely expensive and very inconvenient when intravenous administration is required. (27)
“Drugs developed by the pharmaceutical industry have thus far been associated with toxicity and side effects, which is why natural substances are of increasing interest”(28)
“Current Western therapies for inflammatory diseases are suboptimal; increasingly, patients are turning to complementary and alternative medicine for symptom relief and improved quality of life”(29)
 Functional medicine treatment of AI disease focuses on removing the triggers promoting the autoimmunity and managing the autoimmunity by modulating the immune response and decreasing tissue destruction. It also emphasizes patient education in order to minimize flare-ups and enhance the patient’s ability to recover from the flare-ups when they occur.
Functional medicine treatment of AI disease focuses on removing the triggers promoting the autoimmunity and managing the autoimmunity by modulating the immune response and decreasing tissue destruction. It also emphasizes patient education in order to minimize flare-ups and enhance the patient’s ability to recover from the flare-ups when they occur.
“There is emerging evidence that many of these (complementary and alternative) therapies have the ability to modulate the immune system and disrupt the proinflammatory cascade through a variety of mechanisms, including antioxidant effects, alterations in cell signaling (in particular the nuclear factor (NF)-kB pathway), cytokines, proinflammatory mediators, and disruption of bacterial flora”(30)
“Finding therapeutic agents which can modulate the inflammatory reaction is the highest priority in medical research today”(31)
 There is no cure for autoimmune disease. The primary goal in the management of AI conditions in the functional medicine model is to decrease flare ups, decrease destruction of tissue and increase one’s ability to recover from these flare ups. This can have significant impacts on the quality of life of those with AI disease.
There is no cure for autoimmune disease. The primary goal in the management of AI conditions in the functional medicine model is to decrease flare ups, decrease destruction of tissue and increase one’s ability to recover from these flare ups. This can have significant impacts on the quality of life of those with AI disease.
In the next issue, we will discuss nutritional strategies used to manage autoimmune disease in functional medicine, including dietary and lifestyle strategies and the use of key supplements targeted to the major areas of autoimmunity and autoimmune reactions as identified by the most current literature and described in this article. Stay tuned!
Content provided by Datis Kharrazian, D.C., The Gluten, Leaky Gut, Autoimmune Connection. Apex Energetics lecture. Mar. 16, 2013.
References:
- https://www.aarda.org/q_and_a.php
- https://www.aarda.org/autoimmune_statistics.php
- NAT CLIN PRAC GASTRO & HEP SEPT 2005 VOL 2 NO 9
- Arch Dis Child 1998;79:448-451
- Expert Opin. Med. Diagn. (2008) 2(6):1-13
- Expert Opin. Med. Diagn. (2008) 2(6):1-13
- Arch Dis Child 1998;79:448-451
- Kharrazian, D. The Gluten, Leaky Gut, Autoimmune Connection. Apex Energetics lecture. Mar. 16, 2013.
- Nuclear factor-kB—a pivotal transcription factor in chronic inflammatory diseases. NF-kB Inflammatory Amplifying Loop. N Eng J Med. 1997;336:1066-1071.
- Nuclear factor-kB—a pivotal transcription factor in chronic inflammatory diseases. NF-kB Inflammatory Amplifying Loop. N Eng J Med. 1997;336:1066-1071.
- Return to homeostasis: downregulation of NF-kB responses. Nat Immunol. 2011 Jun 19;12(8):709-714
- NF-kB in type 1 diabetes. Inflamm Allergy Drug Targets. 2011 Jun;10(3):208-217.
- Antiviral and immunomodu-latory properties of new pro-glutathione (GSH) molecules. Curr Med Chem. 2006:13(15): 1749-55
- Correlation of lipid peroxidation and glutathione levels with severity of systemic lupus erythmatosus: a pilot study from single center. J Pharm Pharm Sci. 2008;11(3):30-4
- Intestinal barrier function in response to abundant or depleted mucosal glutathione in Salmonella-infected rats. BMC Physiol. 2009 Apr 17:9:6
- The role of the glutathione antioxidant system in gut barrier failure in a rodent model of experimental necrotizing enterocolitis. Surgery. 2004 Sep;136(3):557-66
- Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005 Sep;2(9): 416-22
- Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms Ann N Y Acad Sci. 2009 May;1165:195-205
- Koshland DE Jr. The molecule of the year. Science. 1992 Dec 18;258(5090):1861.
- Role of nitric oxide in inflammatory conditions. Nephron. 2002 Apr;90(4):373-8.
- Regulatory role of nitric oxide on monocyte-derived dendritic cell functions. J Interferon Cytokine Res. 2003 Aug;23(8):423-31
- Role of nitric oxide in the regulation of T-cell functions. Ann Rheum Dis. 2006 Nov;65 Suppl 3:iii37-40
- Protective role of the cytokine-inducible isoform of nitric oxide synthase induction and nitrosative stress in experimental autoimmune encephalomyelitis of the DA rat. J Neurosci Res. 2003 Jul 15; 73(2):198-205
- Immunoregulatory role of nitric oxide in Kilham rat virus-induced autoimmune diabetes in DR-BB rats. J Immunol. 2004 Jul 15;173(2):1327-35
- Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013 Aug 15;9(1):30. [Epub ahead of print]
- Penn H. Biologic therapies in autoimmune diseases. Clin Med. 2006 Jan-Feb;6(1):105-8. Department of Rheumatology, Royal Free Hospital, London.
- Rosman Z, Shoenfeld Y, Zandman-Goddard G. Biologic therapy for autoimmune diseases: an update. BMC Med. 2013 Apr 4;11:88. doi: 10.1186/1741-7015-11-88.
- J OF PAR AND ENT NUTRITION Vol. 30,no.1, 2006,45-51
- Nutrition in Clinical Practice 23:4962, Feb 2008
- Nutrition in Clinical Practice 23:4962, Feb 2008
- J OF PAR AND ENT NUTRITION Vol. 30, no.1, 2006,45-51